New biomarkers for cancer therapies derived from the research could usher in superior clinical laboratory diagnostics that identify a patient’s suitability for personalized drug therapies and treatments
In another advancement toward accurate precision medicine, Swiss researchers from the University Hospitals of Zurich and Basel, ETH Zurich, the University of Zurich, and pharmaceutical company Roche have developed a multi-omic tumor profiling technology for cancer patients they hope will isolate biomarkers that allow doctors to tailor drug therapies to individual patients’ medical needs.
Once approved for clinical use, not only would these biomarkers become targets for specific cancer therapies, they also would require development of new diagnostic tests that anatomic pathologists could use to determine whether a biomarker was present in a patient.
If yes, the drug can be administered. If no, the patient is not a candidate for that drug. Thus, this research may produce both diagnostic biomarkers and therapeutic targets.
The researchers published their study in the journal Cancer Cell, titled, “The Tumor Profiler Study: Integrated, Multi-omic, Functional Tumor Profiling for Clinical Decision Support.”
Relevance of In-Depth Tumor Profiling to Support Clinical Decision-Making
In the Swiss “Tumor Profiler” (TuPro) project, the research team is examining the cellular composition and biology of tumors of 240 patients with melanoma, ovarian cancer, and acute myeloid leukemia. Recruitment for the study began in 2018. Today, the melanoma cohort is fully enrolled, and the ovarian cancer and acute myeloid leukemia cohorts are nearing complete enrollment.
“The Tumor Profiler Study is an observational clinical study combining a prospective diagnostic approach to assess the relevance of in-depth tumor profiling to support clinical decision-making (“fast diagnostic loop”) with an exploratory approach to improve the biological understanding of disease (“exploratory science loop”),” the TuPro website states.
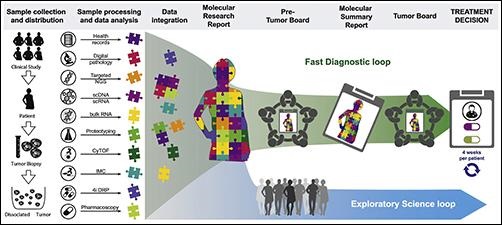
The graphic above taken from the Tumor Profiler project paper illustrates how the TuPro study’s workflow entails patient enrollment, sample collection, analysis by different technology platforms, and data integration, creation and discussion of molecular research and summary reports, discussion of treatment options in pre-tumor boards and the final treatment decision in tumor boards. (Photo copyright: Cancer Cell.)
“For this study of melanoma, ovarian carcinoma, and acute myeloid leukemia tumors, in addition to the emerging standard diagnostic approaches of targeted NGS panel sequencing and digital pathology, extensive characterization is performed using the following exploratory technologies: single-cell genomics and transcriptomics, proteotyping, CyTOF, imaging CyTOF, pharmacoscopy, and 4i drug response profiling (4i DRP),” the TuPro website explains.
In their published paper, the Swiss researchers say these three cancers were selected for the study “based on the potential clinical benefit and availability of sufficient tumor material for simultaneous analysis across all technologies.”

According to a University Hospital Basel blog post, the TuPro project examination of each cancer tumor goes “much further than the limited use of molecular biological methods” used by leading hospitals. “This results in huge amounts of data per patient, which we process and analyze using data science methods,” stated data scientist Gunnar Rätsch, PhD (above), Professor for Biomedical Informatics at ETH Zurich and one of the study’s corresponding authors, in the blog post. This research could lead to new precision medicine biomarkers for clinical laboratory cancer diagnostics and therapies. (Photo copyright: ETH Zurich.)
The TuPro Project’s findings are available to doctors who analyze them at interdisciplinary tumor board meetings and generate treatment options, creating a “fast diagnostic loop” with an estimated four-week turnaround time from surgery to tumor board. “This approach has the potential to alter current diagnostics and paves the way for the translation of comprehensive molecular profiling into clinical decision-making,” the study’s authors wrote in Cancer Cell.
Could Oncologists Be Making Better Precision Medicine Decisions?
In its writeup on the TuPro Project’s research, Precision Oncology News concluded that the Swiss study “is rooted in the researchers’ notion that oncologists are not making the best personalized treatment decisions for patients by relying just on targeted DNA profiling using next-generation sequencing and digital pathology-based tests.
“The researchers within the TuPro consortium hypothesized that integrating a more comprehensive suite of omics tests could lead to a more complete understanding of patients’ tumors, including providing insights into the tumor microenvironment, heterogeneity, and ex vivo responses to certain drugs. This, in turn, could help inform the best course of treatment,” Precision Oncology News added.
“With the Tumor Profiler study, we want to show that the widespread use of molecular biological methods in cancer medicine is not only feasible, but also has specific clinical benefits,” said TuPro consortium member Viola Heinzelmann-Schwarz, MD, Head of Gynecological Oncology at University Hospital Basel, in an ET Zurich news release.
New Precision Medicine Biomarkers from TuPro’s Molecular Analysis
Researchers in the study also are investigating whether and what influence the molecular analysis had on doctors’ therapy decisions.
The University Hospital Basal blog notes the long-term benefits of the Tumor Profiler approach is to expand the personalized-medicine therapy options for patients, including determining whether patients would benefit in certain cases “if they were not treated with drugs from standard therapy, but with drugs that have been approved for other types of cancer.”
Anatomic pathologists and clinical laboratory scientists will want to take note of the TuPro project’s ultimate success or failure, since it could usher in changes in cancer treatments and bring about the need for new diagnostic tests for cancer biomarkers.
—Andrea Downing Peck
Related Information
Swiss Study to Prospectively Assess Value of Multi-Omic, Functional Tumor Profiling



