Mitchell Cancer Institute in Alabama Combines New Robotic Method for Detecting and Excising Biopsies with Rapid On-site Evaluation (ROSE) to Speed Diagnosis of Lung Cancer
Combining robotic-assisted bronchoscopy with rapid on-site evaluation by cytopathologists enables cancer evaluation and diagnosis in one procedure
New technologies are making it possible to both collect a tissue biopsy and diagnose lung cancer during the same procedure. Cytopathologist are essential in this unique approach, which has the potential to greatly shorten the time required to diagnose lung cancer.
At USA Health Mitchell Cancer Institute in Alabama, a team consisting of pulmonology, pathology, surgical, and medical oncology specialists can diagnose lung cancer significantly faster thanks to the combining of a robotic-assisted bronchoscopy (RAB) system with rapid on-site evaluation of biopsies (ROSE) by a cytopathologist during the same procedure.
The RAB platform was created by Auris Health in Redwood City, Calif. According to a USA Health new release, the Auris Health Monarch “enables physicians to see inside the lung and biopsy hard-to-reach nodules using a flexible endoscope. When combined with rapid on-site evaluation (ROSE) it allows for diagnosis at the time of bronchoscopy.”
USA Health says it is the only academic health system in Alabama to combine the Auris Health Monarch (Monarch) with ROSE to diagnose lung cancer in a single procedure.
“Nine-nine percent of the time we make a diagnosis—negative or positive (at time of bronchoscopy). We don’t have to do repeat procedures,” said Elba Turbat-Herrera, MD, Director of Pathological Services at USA Health’s Mitchell Cancer Institute (MCI) and Professor, MCI Interdisciplinary Clinical Oncology, in an exclusive interview with Dark Daily.
The American Society for Cytopathology defines ROSE as “a clinical service provided for patients where a pathologist, or in certain settings, an experienced and appropriately qualified cytotechnologist provides immediate real‐time evaluation of a fine needle aspiration (FNA) biopsy or touch imprints of a core biopsy.”
As a cytopathologist, Turbat-Herrera performs ROSE during procedures at USA Health. “I think we have improved diagnostics very much. With the Monarch equipment, one can see where the needle is traveling in the bronchial tube. It is more precise,” Turbat-Herrera explained.
Patients Benefit from Robotic-assisted Bronchoscopy
Traditionally, anatomic pathologists receive core (tissue sampling) biopsies and fine-needle aspiration biopsies from doctors looking to determine if a lung nodule may be cancerous. But the procedures to secure the biopsies are invasive and stressful for patients waiting for results from clinical laboratories. And some nodules are difficult for surgeons to reach, which can delay care to patients.

“The Monarch and ROSE technologies represent a huge step forward in lung bronchoscopy. Being able to see directly inside the lung and evaluate samples immediately provides the most advanced care for patients,” said Brian Persing, MD (above), Medical Oncologist, Mitchell Cancer Institute, and Assistant Professor of Interdisciplinary Clinical Oncology at the University of South Alabama College of Medicine, in the news release. (Photo copyright: University of South Alabama.)
Currently, more than 112 US healthcare providers use the Monarch robotic-assisted bronchoscopy (RAB) platform, which garnered US Food and Drug Administration (FDA) clearance in 2018, the USA Health news release noted.
The Monarch platform, according to USA Health, “integrates robotics, micro-instrumentation, endoscope design, and data science into one platform to empower physicians.”

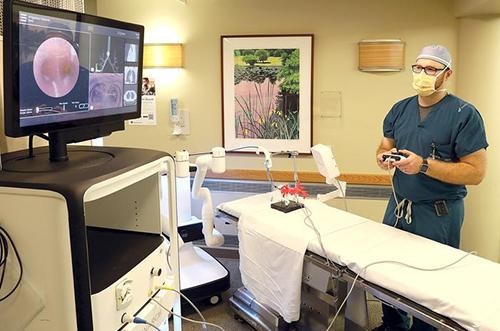
Monarch’s “controller-like interface” (seen above) enables physicians to operate the endoscope and access small and “hard-to-reach” lung nodules. “The Monarch platform,” Duluth News Tribune explained, “is an endoscope guided by a handheld controller very similar to an Xbox controller. As the Monarch Platform drives through the lungs, the camera and other diagrams on a screen help the physician locate the nodule, then collect the biopsy with better accuracy and precision.” (Photo copyright: Jed Carlson/Superior Telegram/Duluth News Tribune.)
Eric Swanson, a pulmonologist at Essentia Health-St. Mary’s Medical Center in Duluth, MD, calls Monarch a game changer. “It’s a big, big upgrade from what we had before,” Swanson told the Duluth News Tribune. “(Before), you’d just pass a small catheter through a regular bronchoscope, and you turn it and hope you land in the right spot.”
The Monarch platform has enabled USA Health to step-up diagnosis of lung cancer, as compared to FNA (fine needle aspiration) biopsy on its own, according to Turbat-Herrera.
“With FNA alone, you try to get (sample tissue), and you are not sure. Now, if it is there, you should get it because the (Monarch) equipment helps you get there. Our role in pathology is to help guide the hand of the pulmonologist: ‘you don’t have what we need,’ or ‘keep going in that area of the lung,’” she said, adding that physicians have been able to reach tiny lesions.
High Incidence of Lung Cancer
The American Cancer Society, says lung cancer is the second most common cancer, with an estimated 235,760 new lung cancer cases and 131,880 deaths from the disease in 2021.
It’s hoped that healthcare providers’ investment in new robotic technology—such as Monarch and others—may shorten the time required to diagnose lung cancer and eventually save lives.
Providers such as USA Health go a step further by integrating ROSE with RAB. The robotic technology—coupled with on-site rapid evaluation by a cytopathologist that averts repeat biopsy procedures—immediately secures an assessment of sample adequacy and a cancer diagnosis that may benefit patients as well.
This is yet another example of how a new technology in one field can have a benefit for anatomic pathologists.
—Donna Marie Pocius
Related Information:
USA Health Mitchell Cancer Institute Offers State-of-the-Art Lung Cancer Diagnosis
FDA Clears Auris Health’s Robotic Monarch Platform for Endoscopy



