Clinical Laboratory Conferences Continue to Tackle COVID-19 Protocols to Put Attendees at Ease
Proof of vaccination, masking, and availability of on-site testing will continue to be measures taken at in-person events for pathologists and medical laboratory professionals
Organizers of in-person clinical laboratory conferences face an interesting dilemma as they plan events in 2022: Where do they draw the line with COVID-19 safety protocols?
On one hand, the surge of cases caused by the SARS-CoV-2 Omicron variant seems to be in its waning stages and large swaths of the population are vaccinated. On the other hand, clinical laboratory and anatomic pathology events want potential registrants to have confidence that it is safe to travel and attend the gatherings.
One lab industry conference producer who happens to be knee-deep in preparing for an in-person meeting this spring is Robert Michel, Editor-in-Chief of The Dark Report and Founder of the 27th Annual Executive War College on Laboratory and Pathology Management. This informative event takes place on April 27-28 in New Orleans and includes COVID-19 protocols to protect attendees.
“It’s important for all those planning to attend this year’s Executive War College to know that screening COVID-19 protocols will be in place to ensure the health and safety of all participants,” Michel noted. “We did a large lab conference in the fall of 2021 that included protocols for COVID-19 and the attendees told us they appreciated the protection provided by those protocols.”
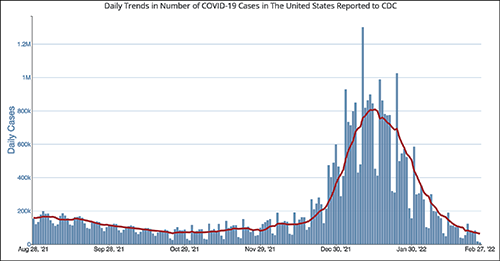
After a significant rise in COVID-19 cases in January 2022 due to the Omicron variant, current daily case levels now are lower than they were six months ago before the new variant hit, according to numbers from the federal Centers for Disease Control and Prevention (CDC).
The in-person 2021 Executive War College, which took place in San Antonio on Nov. 2-3, 2021, followed the CDC’s recommendations:
- COVID-19 protocols included a daily set of questions and a temperature check for all speakers and attendees before they were allowed to enter the conference area.
- CLIA-complex rapid PCR COVID-19 tests were available for individuals whose temperature and answers to the screening questions indicated the need for such testing.
- Attendees used an app to answer the daily screening questions and upload proof of vaccination.
“At last fall’s Executive War College, approximately 400 attendees were screened on each of the three days before entering the conference area and not one rapid COVID-19 test was needed,” Michel said. “Not only is that an outstanding outcome, but a number of attendees also told us they appreciated our efforts to keep them safe and protect their health.”
The 2022 Executive War College will follow the CDC’s updated COVID-19 guidelines, along with any state and local directives in effect as of April 27.

Proof of Vaccination Has Been Required at Other Clinical Lab Industry Events
Organizers of other clinical lab conferences also have dealt with COVID-19 safety protocols. For example, the American Clinical Laboratory Association (ACLA) will hold its annual meeting in Washington, D.C., on March 9. COVID-19-related requirements for attendees will include proof of vaccination uploaded to a vaccine verification vendor and proof of a negative PCR test taken within 72 hours prior to the event.
The annual meeting of the American Society of Clinical Pathology (ASCP) occurs later this year in September in Chicago—too early yet to publish protocols. Last year’s ASCP conference in Boston was a hybrid event, offering both in-person and virtual options. Those who attended in person needed to upload proof of vaccination to a third-party vendor and were required to wear masks. On-site COVID-19 testing was available.
Revived Corporate Travel Could Boost Clinical Laboratory Conferences
The path back to live events across all industries has not been easy given various COVID-19 surges, political divisiveness over masking, frozen corporate travel budgets, and corporate policies banning or limiting employee travel.
Conference organizers throughout the United States universally hope those barriers will lower as 2022 progresses.
“With the fast-spreading Omicron triggering another round of setbacks to start 2022, event planners now are betting on spring to finally mark a turning point for the hard-hit industry,” MarketWatch reported on Feb. 4. “Their hopes hinge on American corporations taking a note from the recovery already under way for domestic air travel for leisure purposes, with the linchpin being a robust revival of trade show attendance and other in-person business gatherings.”
For Michel, offering actionable advice through well-thought-out sessions has been a cornerstone of the content offered each year at the Executive War College. He believes that approach will continue to be the strongest drawing point for clinical laboratory and pathology executives now considering attending the event.
“Our reading of the tea leaves is that across the profession of laboratory medicine, a great many managers, administrators, executives, and pathologists want to return to in-person conferences,” Michel noted. “Registrations for our April event are running ahead of 2019, and people tell us that they recognize the changes in healthcare and the lab marketplace because of the pandemic. They want to understand what’s driving current trends, like greater consumer involvement in lab testing and how to get private payers to reimburse claims for COVID-19 and genetic tests, as well as how a growing number of clinical laboratories are incorporating artificial intelligence solutions in both clinical care settings and lab operations.”
Visit the Executive War College website to see the agenda and to register.
—Scott Wallask
Related Information:
New Lab, Pathology Trends at Executive War College 2021
American Clinical Laboratory Association



