Are Clinical Laboratories Prepared to Cope with Outrage Over Surprise Medical Billing? Patient Access Management May Be an Effective Solution
Consumer demand and federal requirements for price transparency affect how clinical laboratories and anatomic pathology groups meet patients’ expectations while navigating complex payer agreementsRegardless of a clinical laboratory’s payer mix and revenue cycle management (RCM) system, the demand for greater price transparency impacts laboratory services just as it does other healthcare services. Addressing new federal policies that support price transparency may require medical laboratory managers to alter how they approach RCM and patient communications.
Patient access management (PAM) is what some early-adopter medical labs and pathology groups are using to respond to these new federal policies and changing patient expectations. PAM can be an effective tool to fulfill complex payer requirements and implement consumer-friendly healthcare services. Not only does this comply with federal guidelines, it helps independent laboratories increase revenue by lowering denial rates.
How and When Clinical Laboratories Should Implement Patient Access Management
Revenue cycle experts say clinical laboratories are in a position to take an active role in the pricing transparency debate.
“If labs don’t control the pricing narrative, someone else will,” stated Walt Williams, Director of Revenue Cycle Optimization and Strategy for Quadax, a firm that has studied revenue trends in healthcare for more than 40 years, in an exclusive interview with Dark Daily.
He says, given these new demands on clinical laboratories and pathology groups, implementing patient access management practices ensures a satisfactory patient and physician experience and reduces the financial risk related to trends in uncollected revenue.
“In this age of increasing consumerism—along with the complex challenges of navigating the payer landscape and pre-empting administrative denials—it’s no wonder independent labs are turning to new patient access technology solutions to avoid leaving money on the table,” Williams said.
Patient access management solutions allow clinical laboratories to:
- obtain accurate patient demographic information,
- verify insurance coverage and eligibility, and
- gain clarity on payer rules regarding prior authorization and medical necessity.
These capabilities enable medical laboratories to secure appropriate reimbursement closer to the date of service. PAM also can provide the ordering-physician with financial counseling and guidelines on a patient’s financial obligation. This would be shared with the patient to help prevent surprise billing.
New Fact of Life for Labs: Patients Are the New Payers
Medical laboratory patient-access representatives must employ proper patient-liability collection techniques before, during, and after each date of service. This has become increasingly challenging as more patients join high-deductible health plans (HDHPs) and take on more financial responsibility. The problem for labs is that meeting the expectations of consumers requires a different toolset than meeting the needs of complex payer requirements.
Additionally, evolving policies in prior authorization, medical necessity, and coding (see, “Labs Get High Denial Rates Under New NCCI Rules,” The Dark Report) are resulting in potential payment traps for patients and known revenue traps for providers and suppliers.
In its research into trends in healthcare access and affordability, the Peterson Center on Healthcare and the Kaiser Family Foundation (KFF) created the Health System Tracker to monitor consumer spending and surprise medical billing, among other points of interest.
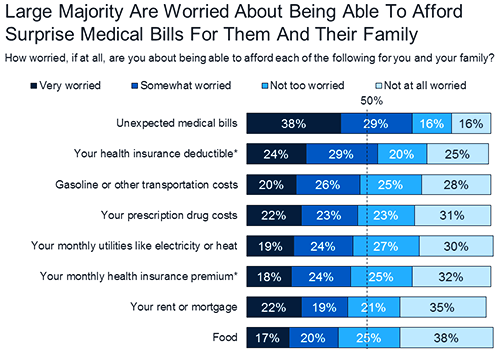
While the current high cost of healthcare will likely continue for some time, publishing information about the lab’s policies can help consumers view choices when it comes to selecting laboratory tests and anticipating potential payment obligations.
Henry Ford Health System, for example, posted information about prior authorization as it relates to its pathology and laboratory services.
Consumer-Facing Price Transparency and CMS Requirements
Rooted in price transparency regulations issued in July 2018, the federal Centers for Medicare and Medicaid Services (CMS) encouraged “all providers and suppliers of healthcare services to undertake efforts to engage in consumer-friendly communication of their charges to help patients understand what their potential financial liability might be for services they obtain, and to enable patients to compare charges for similar services. We encourage providers and suppliers to update this information at least annually, or more often as appropriate, to reflect current charges.”
The questions below, which CMS posed for comment in “Hospital Outpatient Prospective Payment-Notice of Proposed Rulemaking” (CMS-1695-P), may help lab managers tasked with making price transparency program decisions. They include:
- How should we define “standard charges” in provider and supplier settings? Is the best measure of a provider’s or supplier’s standard charges its chargemaster, price list, or charge list?
- What types of information would be most beneficial to patients … enable patients to use charge and cost information in their decision-making?
- How can information on out-of-pocket costs be provided to better support patient choice and decision-making? What can be done to better inform patients of their financial obligations?
- What changes would need to be made by providers and suppliers to provide patients with information on what Medicare pays for a particular service performed by that provider or supplier?
These considerations and more can help the development of patient access management and consumer-friendly communication initiatives that are tailored to clinical laboratory services.
Patient Access Management for Clinical Laboratories
Patient access management facilitates critical components of the revenue cycle. However, it must be fine-tuned to fit each healthcare provider’s unique revenue cycle process. This includes clinical laboratory and anatomic pathology services.
“Having business rules and workflows based on best practices to verify patient demographics, support insurance discovery, and navigate prior authorizations are now a minimum requirement for any healthcare provider to maintain financial viability,” Williams notes.
To help clinical laboratories fulfill CMS’ patient access guidelines—including best practices for reversing the trend of uncollected revenue—a free white paper titled, “Patient Access Antidote: Retaining More Revenue with Front-End Solutions,” has been published by Dark Daily in partnership with Quadax.
The white paper will provide useful insights regarding front-end patient access management. And it will equip clinical laboratories and pathology groups with the expert tools and solutions they need to optimize their cash flow and successfully meet key revenue cycle objectives.
Download it here.
—Liz Carey
Related Information:
WHITE PAPER: Patient Access Antidote: Retaining More Revenue with Front-End Solutions
Undertaking CMS Efforts to Engage in Consumer-Friendly Communication
An Examination of Surprise Medical Bills and Proposals to Protect Consumers from Them



