Dec 10, 2018 | Compliance, Legal, and Malpractice, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Management & Operations, Uncategorized
Clinical laboratory leaders will want to pay close attention to a significant development in Maryland. The state’s All-Payer Medicare program—the nation’s only all-payer hospital rate regulation system—is broadening in scope to include outpatient services starting Jan. 1. The expanded program could impact independent medical laboratories, according to the Maryland Hospital Association (MHA), which told Dark Daily that those labs may see hospitals reaching out to them.
The Centers for Medicare and Medicaid Services (CMS) and the state of Maryland expect to save $1 billion by 2023 in expanding Maryland’s existing All-Payer Model—which focused only on inpatient services since 2014—to also include primary care physicians, skilled nursing facilities, independent clinical laboratories, and more non-hospital settings, according to a CMS statement.
Healthcare Finance notes that it represents “the first time, CMS is holding a state fully at risk for the total cost of care for Medicare beneficiaries.”
Value of Precision Medicine and Coordination of Care to Clinical Labs
“If a patient receives care at a [medical] laboratory outside of a hospital, Maryland hospitals would be looking at ways to coordinate the sharing of that freestanding laboratory information, so that the hospital can coordinate the care of that patient both within and outside the hospital setting,” Erin Cunningham, Communications Manager at MHA, told Dark Daily. Such a coordinating of efforts and sharing of clinical laboratory patient data should help promote precision medicine goals for patients engaged with physicians throughout Maryland’s healthcare networks.
The test of the new program—called the Total Cost of Care (TCOC) Model—also could be an indication that Medicare officials are intent on moving both inpatient and outpatient healthcare providers away from reimbursements based on fees-for-services.
CMS and the state of Maryland said TCOC gives diverse providers incentives to coordinate, center on patients, and save Medicare per capita costs of care each year.

“What they are really doing is tracking how effective we are at managing the quality and the costs of those particular patients that are managed by the physicians and the hospitals together,” Kevin Kelbly, VP and Chief Financial Officer at Carroll Hospital in Westminster, told the Carroll County Times. “They will have set up certain parameters. If we hit those parameters, there could be a shared savings opportunity between the hospitals and the providers,” he added. (Photo copyright: LifeBridge Health.)
The TCOC runs from 2019 through 2023, when it may be extended by officials for an additional five years.
How Does it Work?
The TCOC Model, like the earlier All-Payer Model, will limit Medicare’s costs in Maryland through a per capita, population-based payment, Healthcare Finance explained.
It includes three programs, including the:
- Maryland Primary Care Program (MDPCP), designed to incentivize physician practices by giving additional per beneficiary, per month CMS payments, and incentives for physicians to reduce the number of patients hospitalize;
- Care Redesign Program (CRP), which is a way for hospitals to make incentive payments to their partners in care. In essence, rewards may be given to providers that work efficiently with the hospital to improve quality of services; and,
- Hospital Payment Program, a population-based payment model that reimburses Maryland hospitals annually for hospital services. CMS provides financial incentives to hospitals that succeed in value-based care and reducing unnecessary hospitalizations and readmissions.
CMS and Maryland officials also identified these six high-priority areas for population health improvement:
- Substance-use disorder;
- Diabetes;
- Hypertension;
- Obesity;
- Smoking; and
- Asthma.
“We are going to save about a billion dollars over the next five years, but we are also providing better quality healthcare. So it’s going to affect real people in Maryland, and it helps us keep the whole healthcare system from collapsing, quite frankly,” Maryland Gov. Larry Hogan, told the Carroll County Times.
OneCare in Vermont, Different Approach to One Payer
Maryland is not the only state to try an all-payer model. Vermont’s OneCare is a statewide accountable care organization (ACO) model involving the state’s largest payers: Medicare, Medicaid, and Blue Cross and Blue Shield of Vermont, Healthcare Dive pointed out. The program aims to increase the number of patients under risk-based contracting and, simultaneously, encourage providers to meet population health goals, a Commonwealth Fund report noted.
Both Maryland’s and Vermont’s efforts indicate that payment plans which include value-based incentives are no longer just theory. In some markets, fees-for-service payment models may be gone for good.
Clinical laboratory leaders may want to touch base with their colleagues in Maryland and Vermont to learn how labs in those states are engaging providers and performing under payment programs that, if successful, could replace existing Medicare payment models in other states.
—Donna Marie Pocius
Related Information:
Maryland’s Total Cost of Care Model
Maryland All-Payer Model Expands to Include Outpatient Services
Gov. Hogan Sees Maryland Model as Example for U.S. Healthcare
The Maryland Model
Gov. Larry Hogan, Federal Government Sign Maryland Model All-Payer Contract
CMS Expands Maryland’s All-Payer Program to Outpatient Services
Vermont’s Bold Experiment in Community Driven Healthcare Reform
Nov 30, 2018 | Laboratory Pathology, Laboratory Testing, Managed Care Contracts & Payer Reimbursement, News From Dark Daily
Studies show medical laboratories may be particularly hit by adjustments to hospital chargemasters as hospitals prepare to comply with Medicare’s New Transparency Rule
Recently, Kaiser Health News (KHN) published a story about a $48,329 bill for allergy testing that cast a spotlight on hospital chargemaster rates just as healthcare providers are preparing to publish their prices online to comply with a new Centers for Medicare and Medicaid Services (CMS) rule aimed at increasing pricing transparency in healthcare. The rule goes into effect January 1, 2019.
The patient—a Eureka, Calif., resident with a persistent rash—had received an invoice for more than $3000 from her in-network provider.
Though this type of allergy skin-patch testing is usually performed in an outpatient setting by a trained professional, such as an allergist or dermatologist, the patient elected to have the testing performed at Stanford Health Care (Stanford), a respected academic medical system with multiple hospitals, outpatient services, and physician practices.
The patient’s insurance plan, Anthem Blue Cross (Anthem), paid $11,376 of the $48,329 amount billed by Stanford Health Care, which was the rate negotiated between the insurer and Stanford, Becker’s Healthcare reported. The patient ultimately paid $1,561 out-of-pocket.
So, where did that $48,329 in total charges come from? Experts pointed to the provider’s chargemaster. A chargemaster (AKA, charge description master or CDM) lists a hospital’s prices for services, suppliers and procedures, and is used by providers to create a patient’s bill, according to California’s Office of Statewide Health Planning and Development (OSHPD).
Chargemasters note high prices beyond hospitals’ costs and may be considered jumping off points for hospitals to use in invoicing payers and patients, RevCycleIntelligence explained.
Hospital representatives will negotiate with insurance companies, asking them to pay a discounted rate off the chargemaster list. A patient with health insurance accesses care at that negotiated rate and perhaps has responsibility for a share of that amount as well.
However, an out-of-network patient, uninsured person, or cash customer who receives care will likely be billed the full chargemaster rate.
In a statement to KHN, Stanford explained that the California woman’s care was customized and, therefore, costly: “We conducted a comprehensive evaluation of the patient and her environmental exposures and meticulously selected appropriate allergens, which required obtaining and preparing putative allergens on an individual basis.”

Johns Hopkins researchers Ge Bai, PhD, CPA (left), and Gerard Anderson, PhD (right), authored a study published in Health Affairs that shows “Hospitals on average charged more than 20 times their own costs in 2013 in their CT scan and anesthesiology departments.” Hospitals with clinical laboratory outreach programs will want to consider how their patients may respond as new federal price transparency requirements make it easier for patients to see medical laboratory test prices in advance of service. (Photo copyright: Johns Hopkins University.)
Now is a Good Time for Clinical Laboratories to Make Chargemaster Changes
Some organizations, such as the Healthcare Financial Management Association (HFMA), are calling for chargemaster adjustments as part of a comprehensive plan to improve transparency and lower healthcare costs. This falls in line with the new CMS rule requiring hospitals to post prices online starting Jan.1, 2019.
In fact, hospital medical laboratories, which cannot distinguish their services from competitors, may be impacted by the new CMS rule perhaps more than other services, the HFMA analysis warned.
“The initial impact for healthcare organizations, if they have not already experienced it, will be on commoditized services such as [clinical] lab and imaging. Consumers do not differentiate between high and low quality on a commoditized service the same way a physician might, which means cost plays a larger role in consumers’ decision making.” That’s according to Nicholas Malenka, Senior Consultant, GE Healthcare Partners, and author of the HFMA report. He advises providers to do chargemaster adjustments that relate charges to costs of services, competitors’ charges, and national data.
Medical laboratory leaders also may want to take another look at the opportunities and risks for labs suggested in an earlier Dark Daily e-briefing on the Medicare requirement. (See, “Latest Push by CMS for Increased Price Transparency Highlights Opportunities and Risks for Clinical Laboratories, Pathology Groups,” August 8, 2018.)
Are Chargemaster Charges Truly Excessive? Johns Hopkins Researchers Say ‘Yes!’
Most hospitals with 50 beds or more have a charge-to-cost ratio of 4.32. In other words, $432 is charged when the actual cost of a service is $100, according a study conducted by Johns Hopkins University and published in Health Affairs.
The researchers also noted in a news release about their findings titled, “Hospitals Charge More than 20 Times Cost on Some Procedures to Maximize Revenue,” that:
- Charge-to-cost ratios range from 1.8 for routine inpatient care to 28.5 for a CT scan; and,
- Hospitals with $100 in CT costs may charge an uninsured patient or out-of-network patient $2,850 for the service.
“Hospitals apparently markup higher in the departments with more complex services because it is more difficult for patients to compare prices in these departments,” lead author Ge Bai, PhD, CPA, Associate Professor at Johns Hopkins Carey Business School, noted in the news release.
“(The bills for high charges) affect uninsured and out-of-network patients, auto insurers, and casualty and workers’ compensation insurers. The high charges have led to personal bankruptcy, avoidance of needed medical services, and much higher insurance premiums,” co-author Gerard Anderson, PhD, Professor of Health Policy and Management at Johns Hopkins Bloomberg School of Public Health, stated in the news release.
Legal Issues Possible for Hospitals, Medical Laboratories, Other Providers
Still another study published in the American Journal of Managed Care (AJMC) explored the legality of “surprising” uninsured and out-of-network patients with bills at the chargemaster rates. It found that contract law supports market-negotiated rates—not chargemaster rates that do not reflect actual costs or the market.
“Patients and payers should know that they are under no obligation to pay surprise bills containing chargemaster rates, and state attorneys generally can use the law to prevent providers from pursing chargemaster-related collection efforts against patients,” the researchers wrote.
Labs Need to Get Involved
Clinical laboratory leaders in hospitals and health systems are advised to reach out to hospital chargemaster coordinators to ensure the chargemaster, as it relates to the lab, is inclusive, accurate, and in sync with competitive market data. Independent medical laboratories may want to similarly check their chargemasters to see how their lab test prices compare to the prices charged by other labs serving the same community.
—Donna Marie Pocius
Related Information:
That’s a Lot of Scratch: The $48,329 Allergy Test
Allergy Tests
Six Things to Know About a Woman’s $48K Allergy Test
The Role of the Hospital Chargemaster in Revenue Cycle Management
Why Your Access Strategy Demands Pricing Transparency
CMS Proposes Changes to Empower Patients and Reduce Administrative Burden
US Hospitals Are Still Using Chargemaster Markups to Maximize Revenue
Hospitals Charge More than 20 Times Costs on Some Procedures to Maximize Revenue
Battling the Chargemaster: A Simple Remedy to Balance Billing for Unavoidable Out-of-Network Care
Latest Push by CMS for Increased Price Transparency Highlights Opportunities and Risks for Clinical Laboratories and Pathology Groups
Nov 26, 2018 | Coding, Billing, and Collections, Instruments & Equipment, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
Despite the widespread adoption of electronic health record (EHR) systems and billions in government incentives, lack of interoperability still blocks potential benefits of digital health records, causing frustration among physicians, medical labs, and patients
Clinical laboratories and anatomic pathology groups understand the complexity of today’s electronic health record (EHR) systems. The ability to easily and securely transmit pathology test results and other diagnostic information among multiple providers was the entire point of shifting the nation’s healthcare industry from paper-based to digital health records. However, despite recent advances, true interoperability between disparate health networks remains elusive.
One major reason for the current situation is that multi-hospital health systems and health networks still use EHR systems from different vendors. This fact is well-known to the nation’s medical laboratories because they must spend money and resources to maintain electronic lab test ordering and resulting interfaces with all of these different EHRs.
Healthcare IT News highlighted the scale of this problem in recent coverage. Citing data from the Healthcare Information and Management Systems Society (HIMSS) Logic database, they note that—when taking into account affiliated providers—the typical health network engages with as many as 18 different electronic medical record (EMR) vendors. Similarly, hospitals may be engaging with as many as 16 different EMR vendors.

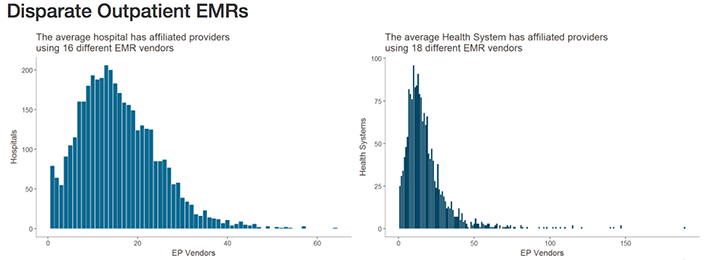
The graphics above illustrates why interoperability is the most important hurdle facing healthcare today. Although the shift to digital is well underway, medical laboratories, physicians, and patients still struggle to communicate data between providers and access it in a universal or centralized manner. (Images copyright: Healthcare IT News.)
The lack of interoperability forces healthcare and diagnostics facilities to develop workarounds for locating, transmitting, receiving, and analyzing data. This simply compounds the problem.
According to a 2018 Physician’s Foundation survey, nearly 40% of respondents identified EHR design and interoperability as the primary source of physician dissatisfaction. It has also been found to be the cause of physician burnout, as Dark Daily reported last year in, “EHR Systems Continue to Cause Burnout, Physician Dissatisfaction, and Decreased Face-to-Face Patient Care.”
Pressure from Technology Giants Fuels Push for Interoperability
According to HITECH Answers, the Centers for Medicare and Medicaid Services (CMS) has paid out more than $38-billion in EHR Incentive Program payments since April 2018.
Experts, however, point out that government incentives are only one part of the pressure vendors are seeing to improve interoperability.
“There needs to be a regulatory push here to play referee and determine what standards will be necessary,” Blain Newton, Executive Vice President, HIMSS Analytics, told Healthcare IT News. “But the [EHR] vendors are going to have to do it because of consumer demand, as things like Apple Health Records gain traction.”
Dark Daily covered Apple’s progress into organizing protected health information (PHI) and personal health records (PHRs) earlier this year in, “Apple’s Update of Its Mobile Health App Consolidates Data from Multiple EHRs and Makes It Easier to Push Clinical Laboratory Data to Patients.” It is one of the latest examples of Silicon Valley tech companies attempting to jump into the health sector and providing patients and consumers access to the troves of medical data created in their lifetime.
Another solution, according to TechTarget, involves developing application programming interfaces (APIs) that allow tech companies and EHR vendors to achieve better interoperability by linking information in a structured manner, facilitating secure data transmission, and powering the next generation of apps that will bring interoperability ever closer to a reality.
TechTarget reported on how University of Utah Hospital’s five hospital/12 community clinic health network, and Intermountain Healthcare, also in Utah, successfully used APIs to develop customized interfaces and apps to improve accessibility and interoperability with their Epic and Cerner EHR systems.
Diagnostic Opportunities for Clinical Laboratories
As consumers gain increased access to their data and healthcare providers harness the current generation of third-party tools to streamline EHR use, vendors will continue to feel pressure to make interoperability a native feature of their EHR systems and reduce the need to rely on HIT teams for customization.
For pathology groups, medical laboratories, and other diagnosticians who interact with EHR systems daily, the impact of interoperability is clear. With the help of tech companies, and a shift in focus from government incentives programs, improved interoperability might soon offer innovative new uses for PHI in diagnosing and treating disease, while further improving the efficiency of clinical laboratories that face tightening budgets, reduced reimbursements, and greater competition.
—Jon Stone
Related Information:
Why EHR Data Interoperability Is Such a Mess in 3 Charts
EHR Incentive Program Status Report April 2018
New FDA App Streamlines EHR Patient Data Collection for Researchers
AAFP Nudges ONC toward EHR Interoperability
A New Breed of Interoperable EHR Apps Is Coming, but Slowly
Top Interoperability Questions to Consider during EHR Selection
EHR Design, Interoperability Top List of Physician Pain Points
2018 Survey of America’s Physicians: Practice Patterns & Perspectives
ONC: 93% of Hospitals Have Adopted Most Recent EHR Criteria, but Most Lag in Interoperability
Open Standards and Health Care Transformation: It’s Finally Delivering on the Value It Promised
Apple’s Update of Its Mobile Health App Consolidates Data from Multiple EHRs and Makes It Easier to Push Clinical Laboratory Data to Patients
EHR Systems Continue to Cause Burnout, Physician Dissatisfaction, and Decreased Face-to-Face Patient Care
Nov 19, 2018 | Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations
New bioinformatic tool finds gut microbiota may be ‘potential reservoir of bloodstream pathogens’ suggesting patients’ own bodies can be source of infections
Clinical laboratories in hospitals and health networks throughout the nation are collaborating in the priority effort to reduce deaths from sepsis and related blood infections. Now comes news that researchers at Stanford have identified an unexpected source of bloodstream infections. This finding may help medical laboratories contribute to faster and more accurate diagnoses of blood infections, particularly for hospital inpatients.
Lax infection-control practices often are blamed for hospital-acquired infections (HAIs). And HAIs certainly have been responsible for many tragic avoidable deaths. However, new research from Stanford University School of Medicine shows that hospital staff, other patients, or unclean instruments may not be solely responsible for all infections that present during hospital stays. According to Stanford researchers, a patient’s own digestive tract can be the surprising culprit for many bloodstream infections. This finding confirms a common belief that the patient’s microbiome probably is involved in many blood infections.
Clinical pathologists have become vital players in infection prevention programs, as hospitals intensify their focus on reducing HAIs. That’s especially in light of the Centers for Medicare and Medicaid Services (CMS) implementation of the pay-for-performance Hospital-Acquired Condition (HAC) Reduction Program. Now, Stanford researchers have found that for many hospital patients their own bodies may be the source of infections.
The researchers published their findings in Nature Medicine.
Bacteria Causing Blood Infections Found in Patients’ Stool Samples After Bone Marrow Transplants
Using a new bioinformatic computational tool called StrainSifter, the Stanford University team rapidly and accurately identified a surprising infection source in a group of hospitalized patients—microbes already living in the patients’ large intestines—a Stanford University news release explained.
The researchers analyzed blood and stool samples from 30 patients who developed bloodstream infections after receiving bone marrow transplants between October 2015 and June 2017 at Stanford Hospital. The researchers sought to determine whether the bacteria isolated from the patients’ blood also was found in stool specimens that had been collected prior to the transplants. The process required sequencing not only the patients’ DNA, but also analyzing the genomes of all the individual microbial strains resident in each patient’s stool.

“Just finding E. coli in a patient’s blood and again in the patient’s stool doesn’t mean they’re the same strain,” Ami Bhatt, MD, PhD, Assistant Professor of Hematology and Genetics at Stanford, explained in the news release. Bhatt served as senior author of the study. (Photo copyright: Stanford University.)
Analysis found that more than one-third of the patients’ stool samples (11) contained detectable levels of the same bacterial strain that had caused those patients’ bloodstream infections.
“Because the gut normally harbors more than 1,000 different bacterial strains, it’s looked upon as a likely culprit of bloodstream infections, especially when the identified pathogen is one known to thrive inside the gut,” Ami Bhatt, MD, PhD, Assistant Professor of Hematology and Genetics at Stanford, said in the news release. “But while this culpability has been assumed—and it’s an entirely reasonable assumption—it’s never been proven. Our study demonstrates that it’s true.”
Clinical and DNA data confirmed the gastrointestinal presence of Escherichia coli and Klebsiella pneumonia, common causes of pneumonia, urinary tract infections, and other potentially serious conditions. In addition, they found other disease-causing pathogens in the gut that they would not have expected to be there.
“We also find cases where typically nonenteric [outside the intestine] pathogens, such as Pseudomonas aeruginosa and Staphylococcus epidermidis, are found in the gut microbiota, thereby challenging the existing informal dogma of these infections originating from environmental or skin sources,” Fiona Tamburini, a senior graduate student, and postdoctoral scholar Tessa Andermann, MD, MPH, Infectious Disease Medical Fellow, wrote in Nature Medicine.
New Tool for Precision Medicine
Bhatt believes being able to trace the source of bloodstream infections will help doctors provide more targeted treatments for HAIs and potentially lead to effective prevention methods. This will create a new opportunity for microbiology laboratories to provide the necessary diagnostic tests designed to guide therapeutic choices of attending physicians.
“Until now, we couldn’t pinpoint those sources with high confidence,” Bhatt said in the news release. “That’s a problem because when a patient has a bloodstream infection, it’s not enough simply to administer broad-spectrum antibiotics. You need to treat the source, or the infection will come back.”
Bhatt says the computational tool has the potential to allow medical practitioners to quickly identify whether a pathogen responsible for a patient’s bloodstream infection came from a break in the skin, leaked through the intestinal wall into the blood, or was passed on through an inserted catheter or other object.
Bhatt’s team focused on the intestines for their study because it’s the home of 1,000 to 2,000 different germs. Dark Daily has reported often on developments involving human gut bacteria (AKA, microbiome) in e-briefings going back to 2013. While these gut bacteria do not typically cause problems, Bhatt said, “It’s only when they show up in the wrong place—due, for example, to leaking through a disrupted intestinal barrier into the bloodstream—that they cause trouble.”
Because nearly 40% of immunocompromised patients who spend up to six weeks in a hospital develop bloodstream infections, the Stanford findings could signal a major breakthrough in preventing HAIs. However, larger studies are needed to validate the researchers’ contention that the gut is a “potential reservoir of bloodstreams pathogens.”
If true, microbiologists and clinical pathologists may in the future have a new method for helping hospitals identify, track, and treat blood-born infections as well as and preventing HAIs.
—Andrea Downing Peck
Related Information:
Study Traces Hospital-Acquired Bloodstream Infections to Patients’ Own Bodies
Hospital-Acquired Condition Reduction Program Fiscal Year 2019 Fact Sheet
Precision Identification of Diverse Bloodstream Pathogens in the Gut Microbiome
Multiple Dark Daily E-briefings on Human Gut Bacteria (Microbiome)
Sep 17, 2018 | Compliance, Legal, and Malpractice, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Managed Care Contracts & Payer Reimbursement, Management & Operations
Ongoing federal regulatory push for EHR interoperability requires medical laboratories and anatomic pathology groups to have strategies for ensuring seamless interfaces with providers and hospitals
What difference does a name make? Clinical laboratories and anatomic pathology groups soon may know the answer to that question following the renaming of the Centers for Medicare and Medicaid Services (CMS) “Meaningful Use” program to “Promoting Interoperability” (PI).
CMS first announced the rebranding in April as part of a proposed rule aimed at transforming the Meaningful Use aspect of the federal Health Information Technology for Economic and Clinical Health (HITECH) Act. HITECH has been Medicare’s roadmap to electronic health record (EHR) implementation and interoperability since it was enacted in 2009.
The final rule arrived on August 2, 2018, and it may impact how clinical laboratories interface with provider and hospital EHRs.
Removing Obstacles to Quality Patient Care
In the news release outlining the updates to Medicare payment policies and rates under the Inpatient Prospective Payment System and the Long-Term Care Hospital Prospective Payment System, CMS states the “overhaul” of the meaningful use program will:
- Make the program more flexible and less burdensome;
- Emphasize measures that require the exchange of health information between providers and patients; and,
- Incentivize providers to make it easier for patients to obtain their medical records electronically.

“We’re excited to make these changes to ensure care will focus on the patient, not on needless paperwork,” CMS Administrator Seema Verma stated in the news release. “We’ve listened to patients and their doctors who urged us to remove the obstacles getting in the way of quality care and positive health outcomes. Today’s final rule reflects public feedback on CMS proposals issued in April and the agency’s patient-driven priorities of improving the quality and safety of care, advancing health information exchange and usability, and removing outdated or redundant regulation on healthcare providers to make way for innovation and greater value.” (Photo copyright: Centers for Medicare and Medicaid Services.)
According to a CMS fact sheet, key provisions of the overhaul include:
- The rule finalized an EHR reporting period to a minimum of any continuous 90-day period in each of calendar years 2019 and 2020 for new and returning participants attesting to CMS or their State Medicaid agency;
- For the Medicare Promoting Interoperability Program, the rule finalized a new performance-based scoring methodology consisting of a smaller set of objectives that CMS states will provide a more flexible, less-burdensome structure, allowing eligible hospitals and critical access hospitals (CAHs) to place their focus back on patients;
- CMS finalized two new e-Prescribing measures related to e-prescribing of opioids (Schedule II controlled substances); and,
- Beginning with an EHR reporting period in CY 2019, all eligible hospitals and CAHs under the Medicare and Medicaid PI programs will be required to use the 2015 Edition of Certified EHR Technology;
- CMS finalized changes to measures, including removing certain measures CMS believes do not emphasize interoperability and the electronic exchange of health information.
According to CMS, about 3,300 acute care hospitals and 420 long-term care hospitals will be subject to the final rule, which takes effect October 1. Obviously, medical laboratories servicing these healthcare organizations will be similarly affected.
Rebranding More than a Name Change
Healthcare Informatics analyzed the 2,593-page final rule explaining that the “core emphasis” of the meaningful use overhaul is “on advancing health data exchange among providers.”
The initial proposal in April, according to Healthcare Informatics, invited stakeholder feedback through a request for information on the possibility of revising CMS’ “Conditions of Participation” for hospitals by requiring providers to electronically transfer medically necessary information following a patient discharge or transfer. The final rule, however, did not include that change.
Instead, the CMS Fact Sheet on the rule states the April request for information was “to obtain feedback on positive solutions to better achieve interoperability, or the sharing of healthcare data between providers, which will inform next steps in advancing this critical initiative.”
Rebranding meaningful use is CMS’s first step in implementing core pieces of the Administration’s MyHealthEData Initiative to strengthen interoperability. In remarks during the ONC Interoperability Forum in Washington, DC, CMS Administrator Seema Verma described the rebranding decision as “much more than a name change” and signaled future CMS actions.
“It is a change in direction for the programs—from programs that support the adoption of health IT, to programs that promote interoperability and patient access to data,” she explained. “To avoid payment reductions and gain incentives, doctors and hospitals will have to give patients electronic access to their health records. We are also considering whether CMS should require—as a condition of participation in the Medicare program—that providers share data with patients in a universal electronic format and hope to share more information on that soon.”
The recent changes follow passage of the Bipartisan Budget Act of 2018, which included a provision relaxing meaningful-use requirements. Though the legislation affects only hospitals and outpatient Medicaid providers, Robert Tennant, Director of Health Information Technology Policy for the Medical Group Management Association (MGMA), declared the revision a “huge win” for providers.
“I don’t think the government recognized how difficult it would be to move from stage 1 to stage 2 to stage 3 [meaningful use] requirements and the significant costs involved,” Tennant stated told Modern Healthcare. “We hope that it signals an interest in Congress in having the administration and HHS (Federal Health and Human Services) not make these quality reporting programs so onerous that it results in large swaths of providers not being successful.”
Clinical laboratories and anatomic pathology groups should be aware that interoperability between their laboratory information systems and the EHRs of providers and hospitals continues to be important. Although the term “Meaningful Use” is to be supplanted by “Promoting Interoperability,” the ability to move patient health information seamlessly among providers continues to be a major goal of this country’s healthcare system.
—Andrea Downing Peck
Related Information:
CMS Finalizes Changes to Empower Patients and Reduce Administrative Burden
In Proposed MU Rebranding Rule, CMS Raises the Interoperability Stakes
Fact Sheet: Fiscal Year (FY) 2019 Medicare Hospital Inpatient Prospective Payment System (IPPS) and Long-Term Acute Care Hospital (LTCH) Prospective Payment System Final Rule (CMS-1694-F)
H.R. 1892: Bipartisan Budget Act of 2018
Printable PDF: Final Rule (CMS-1694-F)
Speech: Remarks by Administrator Seema Verma at the ONC Interoperability Forum in Washington, DC
Congress Budget Deal Relaxes Meaningful-Use Requirements
CMS Proposes Changes to Empower Patients and Reduce Administrative Burden
CMS Proposes Meaningful Use Changes to Promote Interoperability









