COVID-19 Triggers a Cash Flow Crash at Clinical Labs Totaling US $5.2 Billion in Past Seven Weeks; Many Labs Are at Brink of Financial Collapse
Limited availability of COVID-19 clinical lab tests is major topic at federal briefings and news stories, yet many of nation’s labs are laying off staff and at point of closing
Cash flow at the nation’s clinical laboratories has crashed, with revenues down by more than $5 billion since early March. This is the biggest financial disaster for the nation’s clinical laboratory industry in its 100-year history and it couldn’t come at a worse time for the American public and the US healthcare system.
At the precise moment when the nation needs clinical laboratories to begin performing millions of tests for SARS-CoV-2, the coronavirus that causes the COVID-19 illness, those same labs are watching their cash flow collapse.
Data from multiple sources gathered by The Dark Report, sister publication of Dark Daily, confirm that—beginning in early March and continuing through last week—clinical laboratories in the United States saw incoming flows of routine specimens decline by between 50% and 60%. During this same time, lab revenue fell by similar amounts.
Clinical Lab Industry Currently Losing $800 to $900 Million Weekly
To give this decline context, the healthcare system spends about $80 billion annually on medical laboratory testing. Thus, labs across the US generated about $1.5 billion in revenue each week during 2019 and into 2020. By April 5, the decline in routine lab specimen volumes reached 55% to 60%. Since then, the clinical lab industry now loses between $800 million and $900 million each week. Total revenue loss from previous levels is already estimated to be $5.2 billion, and it is growing by an additional $800 million to $900 million every week that patients stay away from hospitals and physicians’ offices.
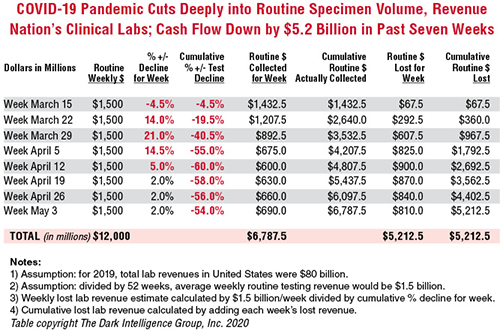
The recent dire financial condition of labs small and large has gone unremarked by federal healthcare officials at the daily White House COVID-19 Task Force briefings. National news sources have yet to report on this development and its implications for successfully expanding the availability and numbers of COVID-19 tests in response to the pandemic.
The rapid and deep decline in specimens and revenue is not limited to clinical laboratories. Biopsy cases referred to anatomic pathology groups have declined by 50% to 60%. Some subspecialty pathology labs saw case referrals drop by 80% or more.
The nation’s two biggest clinical laboratory companies confirmed similar declines in their normal daily flow of routine specimens. Both companies recently reported first-quarter earnings (which included the month of March).
Quest Diagnostics, LabCorp Each Disclose Volume Declines of 50% to 60%
During its Q1 2020 earnings conference call, Chairman, President, and CEO of Quest Diagnostics (NYSE:DGX), Steve Rusckowski, stated, “In April, volume declines continue to intensify as we are seeing signs that volume declines are bottoming out at around 50% to 60%.”
The drop-off in routine lab test referrals was the similar at LabCorp (NYSE:LH). “In our diagnostics business, at the end of the quarter, we experienced reductions in demand for testing of 50% to 55% versus the company’s normal daily levels,” explained Glenn Eisenberg, Executive Vice President and CFO during LabCorp’s Q1 2020 earnings call. “This reduction in demand impacted testing volume broadly but was more heavily weighted towards routine procedures.”
Interviews with independent clinical lab owners and the administrative directors of hospital and health system labs further confirm this rapid and dramatic decline in the number of routine specimens arriving in their labs. Fewer specimens mean fewer claims, which means less revenue to laboratories.
Two Different Financial Futures for ‘Have’ Labs and ‘Have Not’ Labs
What happens next to the clinical laboratory industry in the United States—and to its ability to continue ramping up the availability of adequate numbers of COVID-19 tests in major cities, small towns, and rural areas—will be a story of “haves” and “have nots.”
The “haves” are clinical labs that have access to money. These are publicly-traded lab companies, academic medical center labs, and the sophisticated labs of health networks that operate multiple hospitals. In each case, these organizations have capital reserves and access to loans that will probably enable them to sustain COVID-19 lab testing services at the large volumes required to respond to the pandemic.
Examples of “have” labs would range from public lab companies like LabCorp, Quest Diagnostics, Sonic Healthcare USA, and BioReference Laboratories to the labs of healthcare organizations such as Mayo Clinic, Cleveland Clinic, Geisinger Health, Advocate Aurora Health, and ARUP Laboratories.
The “have nots” will be:
- clinical laboratories that are privately-owned;
- clinical labs operated by community hospitals and rural hospitals that were not financially robust before the onset of the pandemic; and,
- specialty lab companies that perform a specific number of proprietary diagnostic tests (and for which demand has collapsed as patients stopped seeing their doctors).
Medicare Led Payers in the ‘Lab Test Price Race to the Bottom’
Prior to the onset of the SARS-CoV-2 pandemic, the finances of the “have-not” labs were already shaky, with many on the verge of filing bankruptcy, closing, or selling to a bigger lab company. Much blame for the deteriorating finances at a large proportion of community lab companies, community hospital labs, and rural hospital labs can be attributed to the deep, multi-year price cuts to the Medicare Part B clinical laboratory fee schedule as mandated by the Protecting Access to Medicare Act of 2014 (PAMA).
Medicare’s multi-year cuts to lab test prices were immediately copied by most state Medicaid programs. During this period, private payers followed Medicare’s lead and enacted their own deep cuts to the prices they paid labs for both routine tests and molecular/genetic tests.
That is why—when the pandemic intensified in early March—the 50% to 60% drop in specimens and revenue that hit these labs starved them of essential cash flow. When polled, the owners and directors of these labs acknowledge layoffs of the majority of their staff in all departments. They also reported substantial delays—both in submitted lab test claims and in getting payment for those claims—because claims-processing departments at the labs and private health insurers are understaffed due to shelter-in-place directives.
COVID-19 Test Revenue Helps Only Labs Performing Those Tests
Revenue from COVID-19 testing is helping certain labs offset the revenue loss from fewer routine specimens. XIFIN, Inc., a San Diego company that provides revenue cycle management (RCM) services for clinical laboratories and pathology groups, analyzed the lab test claims for COVID-19 rapid molecular tests. It determined that labs performing these tests are generating enough revenue from these test claims to equal about 20% of their pre-pandemic revenue.
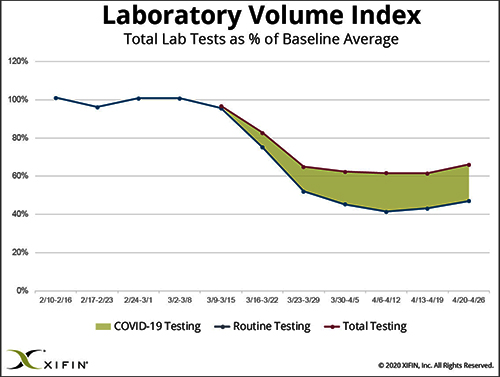
Many CLIA-certified community laboratories and hospital labs have the diagnostic instruments and experience to perform rapid molecular tests for COVID-19. But when contacted, they tell us that their suppliers do not ship them even minimal quantities of the COVID-19 kits, the reagents, and the consumables. Thus, they cannot meet the needs of their client physicians. Instead, they watch as these physicians refer COVID-19 tests to the nation’s largest labs. The supply shortage prevents these smaller labs from doing larger numbers of COVID-19 test for the patients in the communities they serve. It also prevents them from earning the revenues from COVID-19 testing that currently helps the nation’s “have” labs offset the decline in revenue from routine testing.
Congress, national healthcare policymakers, and state governors need to immediately address this situation. Each week that passes during the COVID-19 pandemic and the shelter-in-place directives drains another $800 million to $900 million in revenue from routine lab testing that previously flowed into the nation’s clinical laboratories.
‘Have-not’ Clinical Labs in Small Towns Will Quietly Shrink and Disappear
Without timely intervention and financial support, the nation’s network of ‘have not’ labs, which have so capably served towns away from big metropolitan centers and rural areas, will quietly begin shrinking. One at a time, labs in small towns will close or sell. Local lab facilities will be shuttered and specimens from small-town patients will be transported to big labs hundreds or thousands of miles away.
It is also true that the financial disaster besetting the nation’s clinical laboratory industry will have comparable dramatic consequences for the in vitro diagnostics (IVD) manufacturers that sell them automation, analyzers, reagents, and other supplies. Since early March, IVD manufacturers watched as the pandemic caused orders for new instruments to collapse. During these same weeks, their clinical lab customers ceased ordering routine test kits at pre-pandemic levels. Dark Daily will cover the challenges confronting the IVD and other diagnostics industries in future e-briefings.
Announcing Free COVID-19 STAT Intelligence Briefings for Clinical Labs
With the COVID-19 pandemic creating chaos in nearly every aspect of healthcare, business, and society, clinical labs and their suppliers need timely intelligence and analysis about the innovations and successes achieved by their peers. This week, Dark Daily and The Dark Report are launching COVID-19 STAT Intelligence Briefings (Copy and paste this URL into your browser: https://www.covid19briefings.com). This comprehensive service is free and will cover four basic areas of needs for clinical laboratories as they ramp up COVID-19 testing:
- Daily and weekly COVID-19 testing dashboards to guide every lab’s short-term planning;
- Proven steps for labs to introduce and validate COVID-19 tests (both rapid molecular tests and serology tests);
- Getting paid for COVID-19 testing to ensure every lab’s financial stability and clinical quality; and
- Legal and regulatory updates for labs doing COVID19 tests to ensure full compliance.
Also, to help clinical laboratory leaders deal with the coming wave of COVID-19 serology tests, we are producing a free webinar led by James O. Westgard, PhD, FACB, and Sten Westgard, Director of Client Services and Technology, of Westgard QC, Inc.
“Quality Issues Your Clinical Laboratory Should Know Before You Buy or Select COVID-19 Serology Tests,” will take place on Thursday, May 21, at 1:00 PM EDT. For details and to register, copy and paste this URL into your browser: https://www.darkdaily.com/webinar/quality-issues-your-clinical-laboratory-should-know-before-you-buy-or-select-covid-19-serology-tests.
Each week that the SARS-CoV-2 pandemic continues, and strict shelter-in-place directives are in place, the clinical laboratory industry loses another almost $900 million in revenue from lower volumes of routine testing. No industry can survive when its incoming revenue collapses by 50% to 60% for sustained periods of time.
Will Congress Recognize the Need for a Financial Rescue of ‘Have-not’ Labs?
Thus, it is incumbent on Congress, elected officials, and healthcare policymakers to recognize the financial consequences of the pandemic to the nation’s clinical laboratories. That is particularly true of the ‘have-not’ clinical labs. They do not have the same access to decisionmakers in government as billion-dollar lab companies.
And yet, these labs located in small communities and rural areas often are the only local labs that can do STAT testing in a couple of hours, and where clinical pathologists are personally familiar with local physicians and patients.
These “have-not” labs are vital healthcare resources. They should receive the help they need to get through this unprecedented crisis that is the COVID-19 pandemic.
—Robert L. Michel
Editor-in-Chief
Related Information:
Quality Issues Your Clinical Laboratory Should Know Before You Buy or Select COVID-19 Serology Tests
COVID-19 STAT Intelligence Service: Resources and Help for Labs During the SARS-CoV-2 Pandemic
Is the Coronavirus Antibody Test a Magic Bullet—Or False Hope?



