Department of Justice Recovers $1.8B from Medical Laboratory Owners and Others Accused of Alleged Healthcare Fraud During COVID-19 Pandemic
It did not take long for fraudsters to pursue hundreds of billions of federal dollars designated to support SARS-CoV-2 testing and it is rare when federal prosecutors bring cases only a few months after illegal lab testing schemes are identified
As if the COVID-19 pandemic weren’t bad enough, unscrupulous clinical laboratory operators quickly sought to take advantage of the critical demand for SARS-CoV-2 testing and defraud the federal government.
Unfortunately for the many defendants in these cases, federal investigations into alleged cases of fraud were launched with noteworthy speed. As a result of these investigations into alleged healthcare fraud by clinical laboratories and other organizations during fiscal year (FY) 2020, the US Department of Justice (DOJ) announced the US government has recovered $1.8 billion.
The federal prosecutions involved dozens of medical laboratory owners and operators who paid back “hundreds of millions in alleged federal healthcare program losses,” Goodwin Life Sciences Perspectives explained.
The annual report of the Departments of Health and Human Services (HHS) and Justice Health Care Fraud and Abuse Control Program (HCFAC) reported that federal agencies found and prosecuted alleged healthcare fraud for unnecessary laboratory testing related to:
- COVID-19,
- Genetic sequencing, and
- cardiac panels.
The HCFAC is a joint program of the HHS Office of Inspector General (OIG), Centers for Medicare and Medicaid Services (CMS), and DOJ, a CMS fact sheet explained.
Billions Recovered by HCFAC Program
When combined with similar efforts starting in prior years, the program has returned to the federal government and private individuals a total of $3.1 billion, the DOJ noted.
“In its 24th year of operation, the program’s continued success confirms the soundness of a collaborative approach to identify and prosecute the most egregious instances of healthcare fraud, to prevent future fraud and abuse, and to protect program beneficiaries,” the report states.
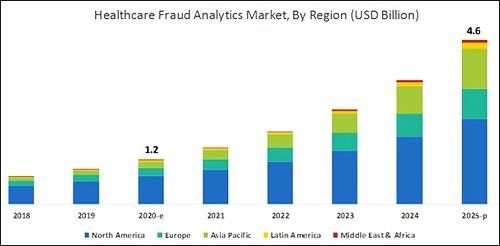
According to the graphic above, which is based on analysis by B2B research company MarketsandMarkets, “North America will dominate the healthcare fraud analytics market from 2020–2025.” As clinical laboratory testing represents a significant portion of the fraud, medical lab managers will want to remain vigilant. (Graphic copyright: MarketsandMarkets.)
COVID-19 Pandemic an Opportunity for Fraud
The HHS report notes that the COVID-19 pandemic required CMS to develop a “robust fraud risk assessment process” to identify clinical laboratory fraud schemes, such as offering COVID-19 tests in exchange for personal details and Medicare information.
“In one fraud scheme, some labs are targeting retirement communities claiming to offer COVID-19 tests but are drawing blood and billing federal healthcare programs for medically unnecessary services,” the HHS report notes.
Still other alleged schemes involved billing for expensive tests and services in addition to COVID-19 testing. “For example, providers are billing a COVID-19 test with other far more expensive tests such as the Respiratory Pathogen Panel (RPP) and antibiotic resistance tests,” the report says.
“Other potentially unnecessary tests being billed along with a COVID-19 test include genetic testing and cardiac panels CPT (current procedural terminology) codes. Providers are also billing respiratory, gastrointestinal, genitourinary, and dermatologic pathogen code sets with the not otherwise specified code CPT 87798,” the report states.
Different Types of Healthcare Organizations Investigated in 2020
Beyond clinical laboratories, the HHS’ 124-page report also shares criminal and civil investigations of other healthcare organizations and areas including:
- clinics,
- drug companies,
- durable medical equipment,
- electronic health records,
- home health providers,
- hospice care,
- hospitals and healthcare systems,
- medical devices,
- nursing home and facilities,
- pharmacies, and
- physicians/other practitioners.
According to the DOJ, “enforcement actions” in 2020 included:
- 1,148 new criminal healthcare fraud investigations opened,
- 440 defendants convicted of healthcare fraud and related crimes,
- 1,079 civil healthcare fraud investigations opened, and
- 1,498 pending civil health fraud matters at year-end.
“Federal Bureau of Investigation (FBI) investigative efforts resulted in over 407 operational disruptions of criminal fraud organizations and the dismantlement of the criminal hierarchy of more than 101 healthcare fraud criminal enterprises,” the DOJ reported.
Furthermore, the report said OIG investigations in 2020 led to:
- 578 criminal actions against people or organizations for Medicare-related crimes,
- 781 civil actions such as false claims, and
- 2,148 people and organizations eliminated from Medicare and Medicaid participation.
Implications for Clinical Laboratories
In 2020, OIG issued 178 reports, completed 44 evaluations, and made 689 recommendations to HHS divisions.
Clinical laboratory leaders may be most interested in those related to patient identification as a means to combating fraud and Medicare Part B lab testing reimbursement.
The HHS report says, “Medicare Advantage (MA) encounter data continue to lack National Provider Identifiers (NPIs) for providers who order and/or refer … clinical laboratory services,” adding that, “Almost half of MA organizations believe that using NPIs for ordering providers is critical for combating fraud.”
Additionally, the report states, “Medicare Part B spending for lab tests increased to $7.6 billion in 2018, despite lower payment rates for most lab tests. The $459 million spending increase was driven by:
- “increased spending on genetic tests,
- “ending the discount for certain chemistry tests, and the
- “move to a single national fee schedule.”
Medical laboratory leaders may be surprised to learn that federal healthcare investigators were so vigorous in their investigations, even during the worst of the COVID-19 pandemic.
Vigilance is critical to ensure labs do not fall under the DOJ’s scrutiny. This HHS report, which describes the types and dollars involved in fraudulent schemes by clinical labs and other providers, could help inform revisions to federal compliance regulations and statutes.
—Donna Marie Pocius
Related Information



