Mar 26, 2018 | Compliance, Legal, and Malpractice, Laboratory Management and Operations, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations, News From Dark Daily
As the public gains awareness of the role clinical laboratories play in modern healthcare, increased engagement and understanding of the technology underlying many of these advances could create risk for labs without transparent reporting protocols to both patients and the public
In recent years, consumers have continually raised the bar in their expectation of quality when they interact with the healthcare system. Not only do patients expect providers—including clinical laboratories and anatomic pathology groups—to improve regularly over time, but the public has even less tolerance for medical errors of any type. Thus, a recent NPR story is one more warning to the medical laboratory profession that it should be devoting resources and effort to improving quality.
Today’s healthcare consumers and patients are more educated about and involved in the care process than ever before. While the exact science and skills may not interest the general public, the technologies underpinning much of the shift toward personalized medicine (AKA, precision medicine) are the same technologies that created the always-connected, digital lifestyles seen around the world.
With this, comes a level of scrutiny and questioning from the public that clinical laboratories or anatomic pathology groups would not have experienced even just a decade ago.
Mounting Scrutiny of Medical Laboratories and Healthcare Professionals
A recent segment on NPR’s “All Things Considered” highlighted this trend and questioned the quality control standards behind many of the procedures powering current testing. The segment also questioned the impact quality control has on the quality of biobanks used to research and create future technologies and tests.
Pathologist Richard Friedberg, PhD, Medical Director of Baystate Reference Laboratories and former president of the College of American Pathologists, told NPR, “We need to be sure that the stuff [doctors and researchers are] looking at is valid, accurate, reliable, and reproducible … If it’s garbage in, it’s garbage out.”
The story highlights improved standards and guidelines surrounding immunohistochemical (IHC) HER2 tests in the early 2000s. In 2007, The New York Times questioned the reliability of the tests, based on studies presented to the American Society of Clinical Oncology the week prior.
In response, the American Society of Clinical Oncology and the College of American Pathologists released guideline recommendations outlining the exact standards required to reduce assay variation and ensure that data produced is accurate and reproducible. NPR’s coverage claims this is the only test with such strict guidelines.
“I don’t think physicians think this way about their entire medical system,” Carolyn Compton, PhD, CMO of the National Biomarker Development Alliance, CMO of the Complex Adaptive Systems Initiative, and professor of Life Sciences at Arizona State University, told NPR. “I don’t see how we’re going to get precision medicine at the end of the day when everything under the hood is so imprecise.”
When data and previous research powers much of the innovation taking place across the modern healthcare landscape, the accuracy of said data would seem critical. Yet, without standards in place, there’s not always safeties by which to verify sample integrity and other critical concerns.
Late last year, Dark Daily reported on a study published in PLOS ONE from Radboud University in the Netherlands questioning the accuracy of more than 30,000 published scientific studies that contained misidentified or contaminated cell lines. Guidelines, such as those created for IHR and FISH HER2 testing, provide standards intended to prevent such issues from occurring or detecting them when they do occur.
Quality versus Quantity: A Gamble Worth Taking?
Apart from challenges with healthcare reform and the regulatory landscape surrounding precision medicine, medical laboratories also must struggle with the challenges of gleaning and maintaining useful, accurate information from an ever-growing trove of data produced by analyzers and assays.
Yet, these mediocre datasets include the results of tests that carried a potentially significant impact on patient lives. In the first two weeks of February alone, both the St. Louis Post-Dispatch and The Telegraph published stories related to erroneous testing related to cancer and the potential impact on the clinical laboratories involved and the patients tested.
Increased coverage shows that the world is watching what goes on in medical laboratories, hospitals, and data centers as healthcare continues to evolve. Clinical laboratories must move forward with this in mind or risk pushback and questioning from the public. Transparency regarding standards, and reporting information to patients surrounding testing or concerns, might effectively address this rising trend.
“We are moving faster and faster and faster as this whole precision medicine train is moving down the track,” Tim Allen, MD, Laboratory Director at the University of Texas Medical Branch Department of Laboratory Services, told NPR. “I suspect standardization of these things is going to become a reality much quicker than I would have expected even a few years ago.”
That quality control issues in anatomic pathology are considered newsworthy by no less than NPR is a sign of increased public attention to the quality of lab testing. The story was written to educate the public about the gap that exists in the quality control of anatomic pathology testing. All of this is consistent with the trend for providers to be transparent and report their quality metrics to the public, including patients.
—Jon Stone
Related Information:
Hormone Receptor Testing Volume 1: Investigation and Findings Commission of Inquiry on Hormone Receptor Testing
Precision Medical Treatments Have a Quality Control Problem
HER2 TESTS: How Do We Choose?
Cancer Drug May Elude Many Women Who Need It
American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer
Impact of Electronic Health Record Systems on Information Integrity: Quality and Safety Implications
His Doctor Said It Was Cancer. It Wasn’t. But the Lab Mix-Up News Came Too Late.
Up to 60,000 Cancer Test Results May Have to Be Reviewed After Women Wrongly Given the All-Clear
Over 30,000 Published Studies Could Be Wrong Due to Contaminated Cells
Netherlands University Researchers Question Validity of More Than 30,000 Published Scientific Studies; Findings Have Implications for Medical Laboratories
Mar 21, 2018 | Digital Pathology, Instruments & Equipment, Laboratory Instruments & Laboratory Equipment, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Sales and Marketing, Laboratory Testing, News From Dark Daily
January’s press release confirmed the tech company is working to integrate critical medical data into its mobile devices, while further promoting interoperability and patient access
While interoperability has improved since the earliest electronic health record (EHR) systems, today’s active patients often need to sort through multiple healthcare portals—including those of clinical laboratories and anatomic pathology groups—to get a comprehensive view of their medical history. Not only can this be time consuming, but also inconvenient if the patient lacks access to a computer.
Thus, it’s no surprise that in a January 24 press release, mobile technology giant Apple announced plans to enter the development ring and create an improved EHR for its mobile device users by updating its existing “Health” mobile application (app). The iOS 11.3 update, among other things, is designed to enable Apple iPhone owners to receive critical medical data, such as medical laboratory test results, directly on their devices.
“Our goal is to help consumers live a better day. We’ve worked closely with the health community to create an experience everyone has wanted for years—to view medical records easily and securely right on your iPhone,” said Apple COO Jeff Williams in the press release.

Jeff Williams (above), COO at Apple, notes that, “By empowering customers to see their overall health, we hope to help consumers better understand their health and help them lead healthier lives.” (Photo copyright: Apple.)
The new features are already available to developers in the latest iOS 11.3 beta 3 release. However, release to the public is expected soon with the issuance of the iOS 11.3 final release. This means that patients will not need to download extra apps—or remember to use them—to take advantage of the feature.
New Way to Improve Patients’ Access to Health Data or Just Another Data Silo?
The Apple Health Records platform adheres to Fast Healthcare Interoperability Resources (FHIR) protocols for transmission of data. Providers send information to Apple which then aggregates the information, transmits it to patients’ iPhones and notifies them of the updates.
All information stored on the device is encrypted in storage and protected from unauthorized access by the user’s password.
Through the new Health Records interface, users view this aggregated data as a timeline, conduct searches, and share information with other parties as they deem appropriate.
Current medical information listed in the press release includes:
- Allergies;
- Conditions;
- Immunizations;
- Clinical laboratory results;
- Medications;
- Procedures; and,
- Vitals.
Currently, the platform integrates data from three major EHR developers:
- Epic;
- Cerner; and,
- AthenaHealth
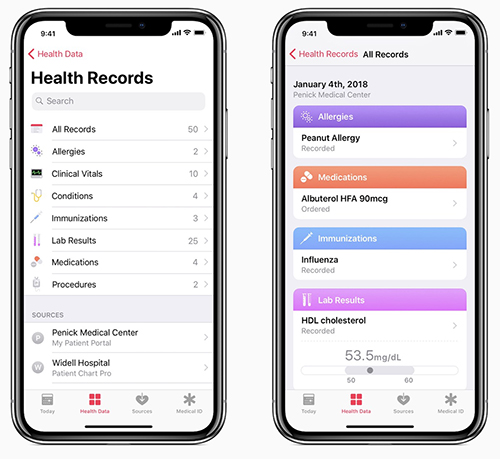
Apple’s update to the Health app makes it easier for people to access and control of all of their health records and data. This included medical laboratory tests. (Image and caption copyright: Apple.)
Apple is also working with 12 health institutions across the US in the first phase of the project, including:
In the Apple press release, Stephanie Reel, CIO at John Hopkins Medicine in Baltimore, stated, “Streamlining information sharing between patients and their caregivers can go a long way towards making the patient experience a positive one. This is why we are excited about working with Apple to make accessing secure medical records from an iPhone as simple for a patient as checking email.”
Previous Attempts at Mobile Health Record Devices Got Mixed Results
This isn’t the first time a major technology company has attempted to enter the mobile health market. Google Health was shuttered in 2011 citing low adoption. Wearable fitness trackers, such as Fitbit (NYSE:FIT) enjoyed a bubble, but are now seeing mixed success in terms of long-term adoption and use, according to The Motley Fool. More to the point, they’ve never quite become the holy grail of monitoring and data collection that some experts predicted, Huffington Post reported.
However, Apple’s investments and interest in healthcare-related technologies has led to wide speculation that they would enter the health market this year. (See Dark Daily “Apple May Be Developing Mobile Device Technology to Monitor User’s Health and Transmit Data in Real Time.”)
Larry Dignan, Editor-in-Chief at ZDNet, builds a compelling case for why this could be the attempt that succeeds in providing a consolidated platform for clinical laboratories, physicians, and other care providers to push data directly to patients and—with the patient’s permission—to each other, regardless of the platforms healthcare facilities use to store and transmit data.
He notes that much of Apple’s newest features build on foundations laid by the healthcare industry to create scalable, functional EHR systems. By working with existing protocols, Apple’s Health Records platform is already positioned for compatibility with many healthcare providers.
Furthermore, Apple is already known for partnering at the enterprise level with major businesses and industries, while also holding the trust of millions of Americans who store their personal information on Apple devices.
Is Apple the Future of EHRs?
Despite this, until the platform—and adoption by the public—is proven a success, it will be yet another walled garden of medical information. Even then, Apple is only one segment of the global mobile market.
Unless Apple provides access to other platforms (such as Android), those patients—and the medical communities serving them—are left consolidating information on their own through a sprawl of various portals. This also means that medical laboratories, pathology groups, and other service providers must continue to invest time and funding into communicating data in ways compatible with a plethora of internal and external systems and software.
Still, the platform offers an intriguing glimpse at the future of medical records and heralds a shift toward empowering patients with easy, comprehensive access to their own data, which would be a boon to the medical laboratory industry.
—Jon Stone
Related Information:
Apple Previews iOS 11.3
Apple Announces Effortless Solution Bringing Health Records to iPhone
With Medical Records Tools, Apple Wades Deeper into Digital Health
Apple Confirms “Health Records” Solution with Aim to Bring Medical Records to iPhone
Apple Will Let You Keep Your Medical Records on Your iPhone
Apple Unveils mHealth Integration with EMR Data through Health App
Apple, Inc. Wants to Solve the Problem of Electronic Health Records
Viewpoint: How Realistic Is Apple’s Attempt at the EHR Industry? Very—6 Reasons Why
Apple Can Win Electronic Medical Record Game with Health Records in iOS 11.3: Here’s 7 Reasons Why
Apple Is Officially in the EHR Business. Now What?
Apple to Launch Health Records App with HL7’s FHIR Specifications at 12 Hospitals
Could Amazon or Apple Actually Make a Dent in the EHR Market?
Apple May Be Developing Mobile Device Technology to Monitor User’s Health and Transmit Data in Real Time
Feb 28, 2018 | Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations, News From Dark Daily
As healthcare reform continues to impact revenues for medical labs and anatomic pathology groups in an effort to reduce healthcare spending, researchers reinforce claims that prices are to blame, not quantity or quality of care
All facets of the US healthcare system—be it massive health systems, medical clinics, independent anatomic pathology groups, or medical laboratories—are experiencing pressure as healthcare reform attempts to manage ever-growing healthcare spending.
Current healthcare trends in the United States focus on determining medical necessity, adopting personalized medicine, and on determining the value various aspects of care. What these trends have in common is a goal of lowering the cost of care while contributing to improved patient care. However, research dating back as far as 2003 suggests overall prices play a significant role in US healthcare spending, particularly when the cost of care in the US is compared to the cost of care in other developed countries.
US Spends More on Healthcare than Any Other Country
A study published last year was the subject of a recent story in the New York Times about why healthcare costs in the United States are so much higher than in other developed nations. The NYT story referenced multiple studies on the subject that all made a similar conclusion: utilization of healthcare in the US is at or below the median compared with other developed nations, and it is higher prices for these services that causes healthcare in the US to be so expensive.
Health Affairs published one such study in 2003, titled, “It’s the Prices, Stupid: Why the United States is So Different from Other Countries.” Written by Gerard F. Anderson, PhD; Uwe E. Reinhardt, PhD; Peter S. Hussey, PhD; and Varduhi Petrosyan, PhD, the paper compared data from the Organization for Economic Cooperation and Development (OECD) for 30 member countries in 2000.
“The data show that the United States spends more on healthcare than any other country. However, on most measures of health services use, the United States is below the OECD median,” researchers state. “These facts suggest that the difference in spending is caused mostly by higher prices for healthcare goods and services in the United States.”

In a New York Times article, Austin Frakt, PhD (above left), Health Economist with the federal Department of Veterans Affairs, and Aaron E. Carroll, MD (above right), pediatrician and Professor of Pediatrics at Indiana University School of Medicine, collated the various research into healthcare spending conducted in the past decade and a half. (Photo copyrights: JAMA/The Accidental Economist.)
“What was true in 2003 remains so today,” Ashish Jha, MPH, a physician with the Harvard T.H. Chan School of Public Health and the director of the Harvard Global Health Institute, told The New York Times (NYT). “The US just isn’t that different from other developed countries in how much healthcare we use. It is very different in how much we pay for it.”
New Data Shines the Spotlight on Old Concerns
Using data spanning from 1996 to 2013, researchers published similar findings in a 2017 JAMA original investigation. “Healthcare spending increased by $933.5 billion from 1996 to 2013,” stated study authors Joseph L. Dieleman, PhD, Assistant Professor at Institute for Health Metrics and Evaluation; Ellen Squires, MPH, Policy Analyst at Kaiser Family Foundation; and Anthony L. Bui, MPH, MD Candidate at David Geffen School of Medicine, UCLA Health. “Service price and intensity alone accounted for more than 50% of the spending increase, although the association of the five factors with spending varied by type of care and health condition.”
The JAMA study authors noted they could not separate price and care intensity in their data. However, as pointed out by NYT, four other studies published by The National Bureau of Economic Research, the OECD, JAMA, and the Annals of Internal Medicine between 2010 and 2017 also link the cost of care directly with healthcare spending in the US.
“The JAMA study found that, together, [care intensity and pricing] accounted for 63% of the increase in spending from 1996 to 2013,” noted The New York Times, “In other words, most of the explanation for American health spending growth—and why it
has pulled away from health spending in other countries—is that more is done for patients during hospital stays and doctor visits, they’re charged more per service, or both.”
For example, the OECD pilot study from 2010 states, “One of the key findings of the pilot study is that the price level of hospital services in the United States is more than 60% above that of the average price level of 12 countries included in the study.”
The More Things Change the More They Stay the Same
As a cornerstone of economics, transparency in both pricing and quality serves to empower buyers—or in this case patients—to choose products and services based on their overall value. This, in turn, encourages competition and helps to keep prices in check.
However, the US healthcare system offers little transparency—either for patient outcomes or for the prices to be charged—with many patients not having any clue what a service will cost until the bill for their recent hospital stay, lab tests, wellness visit, or ER visit arrives. This has led to increased pressure from employers and patient advocates for hospitals, clinical laboratories, and other service providers to make this information available to the public.
Yet, the opposite scenario is the current reality. Lobbyists and groups representing hospitals, insurers, pharmaceuticals, and other facets of the healthcare system continue to promote legislation at the federal and state levels to keep this information private and away from both the public and their competitors.
The NYT highlights the balance surrounding the issue citing claims of increased innovation with higher prices. However, this only works if the market can support said prices. “Though it’s reasonable to push back on high healthcare prices,” NYT’s noted, “there may be a limit to how far we should.”
—Jon Stone
Related Information:
Why the US Spends So Much More than Other Nations on Health Care
Decomposing Medical Care Expenditure Growth
Comparing Price Levels of Hospital Services Across Countries
The Anatomy of Healthcare in the United States
Price and Utilization: Why We Must Target Both to Curb Health Care Costs
Factors Associated with Increases in US Health Care Spending, 1996–2013
It’s the Prices, Stupid: Why the United States is So Different from Other Countries
US Health Care from a Global Perspective
Dec 12, 2017 | Compliance, Legal, and Malpractice, Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations, News From Dark Daily
In filing Monday, lawsuit seeks to force HHS to comply with PAMA’s statutory requirements and to withhold applying the new Clinical Laboratory Fee Schedule until HHS has revised the final rule appropriately
Many clinical laboratory executives will welcome the news that a lab industry trade association has filed a lawsuit in federal court in an effort to delay and fix the final rule for Protecting Access to Medicare Act of 2014 (PAMA) private payer lab test market price reporting that Medicare officials used to lower prices on the Medicare Part B Clinical Laboratory Fee Schedule (CLFS) that is scheduled to take effect on Jan. 1, 2018.
In a lawsuit filed Monday, the American Clinical Laboratory Association (ACLA) charged that the federal Department of Health and Human Services (HHS) ignored congressional intent and instituted a highly-flawed data reporting process when setting the 2018 CLFS rates under the Protecting Access to Medicare Act of 2014.
The ACLA asked the US District Court for the District of Columbia to force HHS to comply with PAMA’s statutory requirements, to withhold applying the new CLFS until HHS has revised the final rule appropriately. The CLFS is due to take effect on Jan. 1.
The lawsuit also seeks to vacate any actions that HHS made that were not in accordance with the PAMA law and to withdraw or suspend the final rule under PAMA. The case is American Clinical Laboratory Association v. Hargan, US District Court, District of Columbia, No. 1:17-cv-2645.
Final Prices for the 2018 Part B Clinical Laboratory Fee Schedule
Last month, the federal Centers for Medicare and Medicaid Services (CMS) issued the final CLFS rates and said at the time that it did so in compliance with the 2016 final rule implementing changes to the Medicare clinical laboratory fee schedule under PAMA section 216.
“We have repeatedly advised CMS that there are significant, substantive deficiencies in the final rule, which fail to follow the specific commands of the PAMA statute,” said ACLA President Julie Khani in an ACLA press release. “Contrary to Congress’ intent, instead of reforming Medicare reimbursement rates to reflect the broad scope of the laboratory market, the Secretary’s final rule will disrupt the market and prevent beneficiaries from having access to the essential laboratory services they need.”

Shown above is Julie Khani, President of the American Clinical Laboratory Association (ACLA) speaking at the Executive War College on Laboratory and Pathology Management last May in New Orleans. In a press release announcing ACLA’s lawsuit against the Department of Health and Human Services, Khani emphasized that many clinical laboratories had advised officials at the federal Centers for Medicare and Medicaid Services (CMS) about the “significant, substantive deficiencies in the final rule” for private payer market price reported that CMS designed. (Photo copyright: The Dark Report.)
22 Healthcare Organizations Opposed Cuts to Clinical Laboratory Test Prices
The ACLA, the American Hospital Association (AHA), and more than 20 other organizations had urged CMS to suspend implementation of the new CLFS rates, which are scheduled to take effect Jan. 1. The organizations cited concerns over the data-collection process used to establish the rates, and the fact that the rates would cause clinical laboratories to struggle financially and possibly close. If the rates set under PAMA affect Medicare beneficiaries’ access to clinical lab testing, the law would have the opposite effect of its intent.
To bring the lawsuit, ACLA retained Mark D. Polston, JD, of the Washington, DC, law firm of King and Spaulding. A specialist in representing healthcare systems seeking to navigate Medicare regulations, Polston is the former Chief Litigation counsel for CMS and specializes in complicated Medicare reimbursement litigation. Recently, he successfully challenged Medicare’s so-called “two-midnight” rule that imposed a 0.2% rate cut on hospitals billing for some patients.
Medicare Program Prohibited Most Medical Laboratories from Reporting
Contrary to Congress’ directives, most laboratories were prohibited from reporting private payer data under CMS’ market-rate data-collection process, ACLA said in a prepared statement. “As a result, CMS failed to protect access to laboratory services for Medicare beneficiaries. This flawed process could cause serious financial harm to potentially thousands of hospitals, independent and physician office laboratories, and make it harder for Medicare beneficiaries to get access to medical testing, particularly in remote rural areas and in nursing homes that depend on laboratory testing services,” ACLA said.
In the lawsuit, ACLA alleged that more than 99.3% of hospitals were prohibited from reporting their market-rate data. It is believed that this is the first time this figure has been reported. In 2015, the lawsuit charged, more than 261,500 entities received Medicare payment for laboratory services but only 1,942 laboratories reported market-rate information in 2016 under the PAMA final rule. The 1,942 labs that reported market-rate data is about 0.7% of the total number of laboratories that serve Medicare beneficiaries, the lawsuit said.
Only 21 of 7,000 Hospital Laboratories Reported Data
“Moreover, contrary to Congress’ intent, the laboratories that did report information are not representative of the market as a whole,” the lawsuit added. “For example, although approximately 7,000 hospital laboratories billed Medicare for laboratory services in 2015—accounting for 24% of the Medicare payments made under the Clinical Laboratory Fee Schedule—no more than 21 hospital laboratories (and probably even fewer) reported information to the secretary, leaving hospital laboratories effectively unrepresented in the data collected by the secretary.
“Hospital laboratories are often the only laboratories available to patients in certain areas of the country, and the private payer rates they receive are often much higher than other laboratories, due to differences in competitive markets, volumes of services, and other factors,” the lawsuit charged.
The Dark Report, Dark Daily’s sister publication, provided a compelling example of the serious flaws in the market price study conducted by CMS. Writing about the state of Michigan, The Dark Report noted: “At Joint Venture Hospital Laboratory Network (JVHL), CEO John Kolozsvary said Michigan’s hospitals serve 70% of the office-based physicians in the state with outreach lab testing services. Included among these hospitals are the 120 JVHL member laboratory facilities.”
“Since our network, plus the outreach programs of another 25 or 30 hospitals, hold a significant share of outreach lab testing in Michigan, how can CMS conduct an accurate, representative market study of what private insurers pay for lab tests in Michigan if it doesn’t collect data on what private payers reimburse hospital lab outreach programs in Michigan?” stated Kolozsvary in his interview with The Dark Report.
Did CMS ‘Disregard and Violate’ PAMA Statute?
In the ACLA’s announcement of the lawsuit, Polston said, “CMS clearly disregarded and violated the statute’s specific, unambiguous directives requiring commercial rate information to be reported and collected from a broad, diverse group of market participants. Instead, information was collected from less than 1% of US laboratories.”
In the press announcement, ACLA Board Chair Curt Hanson, MD, Chief Medical Officer of Mayo Medical Laboratories said, “This lawsuit reflects our obligation to those who are providing critical testing services, and to those millions of Americans who rely on the services our industry provides.” Others supporting the lawsuit include Laboratory Corporation of America and Quest Diagnostics.
Compliance with PAMA Law’s Statutory Requirements
In the lawsuit, ACLA seeks to require HHS to comply with the statutory requirements and to set aside the provisions in the final rule, “that unlawfully exempts thousands of laboratories from the reporting obligations that Congress imposed” under PAMA. A central feature of PAMA Section 216 is that laboratories must report market rate data so that HHS can ensure that Medicare reimbursement rates closely reflect the rates laboratories receive from private payers, the lawsuit said.
“ACLA was a strong supporter of Congress’ market-based reforms, which resulted in the most extensive changes to the system for reimbursing clinical laboratories since 1984,” the lawsuit said.
In challenging the final regulations, the lawsuit said HHS disregarded and violated, “the statute’s specific, unambiguous directives requiring that all applicable laboratories report relevant data.”
Congress Specified Which Medical Laboratories Are Obligated to Report
“In imposing these requirements, Congress took care to specify which laboratories would be obligated to report market data to ensure that information would be collected from a broad, diverse group of market participants,” the lawsuit said. “Congress made clear that any ‘laboratory’ would be required to report data if, ‘with respect to its revenues under [the Medicare program], a majority of such revenues are from’ the Physician Fee Schedule or the Clinical Laboratory Fee Schedule,” the lawsuit charged.
In promulgating the regulations, however, HHS, disregarded Congress’ instructions and “unreasonably and arbitrarily exempted significant categories and large numbers of laboratories that meet the statutory definition from the reporting requirements that Congress imposed,” the lawsuit said.
“The secretary’s final rule fatally undermines one of PAMA’s purposes, which is to require a broad spectrum of Medicare-participating laboratories to report market information to the secretary. Instead, in ultra vires (Latin for “beyond the powers”) fashion, the secretary has carved out large categories of laboratories—ultimately resulting in the exclusion of some 99.3% of the laboratory market—from the statutory reporting requirements,” the lawsuit charged. Ultra vires acts fall outside the authority of the organization in question.
In the lawsuit, the ACLA claims under:
count 1: ultra vires agency action not in accordance with law, in excess of statutory authority;
count 2: unreasonable construction of statute;
count 3: violation of the Administrative Procedure Act, arbitrary and capricious action; and,
count 4: violation of the Administrative Procedure Act, injunctive and declaratory relief.
Seeking an Injunction to Have HHS Secretary to Withhold or Suspend Final Rule
In its final section, “Prayer for Relief,” the lawsuit asks the court to vacate, “any agency action found to be arbitrary, capricious, an abuse of discretion, or otherwise not in accordance with law;” to require the Secretary of HHS to comply with the statutory requirements, “including faithfully implementing the statutory definition of ‘applicable laboratory;’” and enter an “injunction that (1) directs the Secretary to withdraw or suspend his final rule until such time as it can be brought into compliance with the statute, and (2) directs the Secretary to withhold applying the new Clinical Laboratory Fee Schedule until such time as the Secretary has made appropriate revisions to his final rule.” The lawsuit also asked the court to award to the ACLA “costs and disbursements of this action and reasonable attorneys’ fees.”
—Joseph Burns
Related Information:
ACLA Files Lawsuit Challenging PAMA Rates
CMS Ignored Congressional Intent in Implementing New Clinical Lab Payment System Under PAMA, ACLA Charges in Suit
Quest Diagnostics Supports Suit Against HHS Charging That CMS Ignored Congressional Intent in Implementing New Clinical Lab Payment System
LabCorp Supports American Clinical Laboratory Association Lawsuit on PAMA Final Rule
For Top 20 Tests, CMS to Cut Payment by 28% in 2018-2020; Medicare officials move one step closer to destroying beneficiary access to lab tests: The Dark Report, October 9, 2017
Dec 1, 2017 | Laboratory News, Laboratory Pathology, Laboratory Testing, Management & Operations, News From Dark Daily
Professor-led classroom lectures end as students are expected to do much of their traditional learning outside of class. Will this influence how many medical students go on to choose pathology for their residency?
Medical Colleges, hospital universities, and healthcare trade schools nationwide are considering “Active Learning” techniques to replace lectures. These bastions of higher education—where anatomic pathologists, medical laboratory scientists, doctors, nurses, clinical laboratory technicians, and other healthcare professionals learn their skills—are adopting evidence-based teaching styles that resonate with modern technology-savvy students.
In September, the University of Vermont Larner College of Medicine (UVM) became the latest institution to embrace this trend when it announced it would abolish lectures across all of its programs beginning in 2019. This makes UVM the first member of the Association of American Medical Colleges (AAMC) to drop lectures from its curriculum.
“What we know about learning in general is different than it was decades ago,” Lisa Howley, PhD, AAMC Senior Director of Strategic Initiatives and Partnerships, told Inside Higher Education. “Our medical students are of a generation that has grown up differently when it comes to technology and the impact that has on their ability to receive and retain information.”
Dubbed a “flipped classroom,” students do homework before classes rather than after, as would be done in a traditional education setting. They are expected to learn material online and through textbooks, and then complete self-assessments to gauge their understanding of what they’ve learned. Classroom time involves so-called “active learning,” which includes problem-solving in small groups, question-and-answer sessions, and group discussions.
UVM Not First to Drop Lectures
While UVM’s announcement has generated headlines and controversy, it is not the first medical school to abandon traditional lectures. Cleveland Clinic’s Lerner College of Medicine at Case Western Reserve University opened in 2004 with a no-lecture format.
A growing body of research, such as this study published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS), indicates that active learning improves student performances, especially in science, technology, engineering, and mathematics. With the specialties of pathology and medical laboratory medicine heavily dependent on technology and science, this may be a favorable development for medical students who decided to specialize in these fields.
“We teach evidence-based medicine all the time,” William Jeffries, PhD, Senior Associate Dean for Medical Education at UVM, stated in the Inside Higher Education article. “If you have the evidence to show one treatment is better than the other, you would naturally use that treatment. So, if we know that there are methods superior to lecturing, why are we lecturing at all?”

Kelly J. (McDonough) Butnor, MD (center), Surgical Pathologist and Professor of Pathology and Laboratory Medicine at University of Vermont College of Medicine, conducts a team-based learning session with second-year students. (Photo and caption copyright: The Washington Post, Erin Post, Larner College of Medicine.)
In 2112, Charles G. Prober, MD, Senior Associate Vice Provost for Health Education and Professor of Pediatrics at Stanford School of Medicine, and Chip Heath, PhD, Professor of Organizational Behavior in the Stanford Graduate School of Business, called for a “change in the way we educate doctors.”
In “Lecture Halls without Lectures—A Proposal for Medical Education,” published in the New England Journal of Medicine (NEJM), Prober and Heath wrote, “Students are being taught roughly the same way they were taught when the Wright brothers were tinkering at Kitty Hawk.” They suggested five years ago that active learning and short online videos were more effective and a better use of students’ limited time than auditorium-style mandatory lectures. Today, with mobile technologies and streaming Internet technologies, their argument is even more valid.
Lack of Funds Blocks Innovation
Jeffries contends the cost of making wholesale changes in how students are taught, which requires retraining faculty and renovating classrooms, keeps most medical schools from overhauling teaching methods. “Most schools do not have the resources to turn the battleship around,” he told Inside Higher Education.
At UVM, however, a $66-million gift last year by Robert Larner, MD, and his wife Helen, is helping fund the school retrain its medical school teaching staff and redesign classroom spaces to support active learning. Larner is a dual-degree alum whose name now adorns the medical school.
In a recent NEJM article, Richard M. Schwartzstein, MD, Professor, Beth Israel Deaconess Medical Center (BIDMC) at Harvard Medical School, and David H. Roberts, MD, Dean for External Education at Harvard Medical School, point out that “the movement away from traditional lecture-based courses has been under way in US medical schools for more than three decades.” They question, however, whether the push to do away with all lectures is “merely the latest fad in medical education” or is it truly evidence-based?
“We can often serve our students best by fusing elements of various methods, such as team-based or case-based learning and interactive large-group learning sessions, rather than feeling obliged to adhere to a particular format,” they wrote. “But we must also use evidenced-based approaches whenever possible and rigorously evaluate our innovations, acknowledging that important outcomes may include student engagement and problem-solving skills, team dynamics, and the learning environment as much as exam scores.”
Prober told the Washington Post that medical school students already vote with their feet for the type of teaching format they prefer.
“When you go into a lecture in medical schools across the nation, you will find a minority of students actually present,” he said. “Medical students are adults. One generally believes adults try to make decisions that are in their best interests. They have seemingly made the decision that it is not in the lectures.”
For the past two decades, many pathologists have regularly pointed out that advances in technologies and procedures in both anatomic pathology and clinical laboratory medicine have outpaced the ability of medical schools and residency programs to incorporate these new developments into training programs. Thus, clinical laboratory scientists and pathologists will be watching with interest to see if these new models for medical school education are capable of incorporating new advances in laboratory medicine into their training formats in a timely fashion.
—Andrea Downing Peck
Related Information:
Become a Doctor, No Lectures Required
Medical School Without the ‘Sage on a Stage’
Active Learning Increases Student Performance in Science, Engineering, and Mathematics
Lecture Halls without Lectures–A Proposal for Medical Education
Saying Goodbye to Lectures in Medical School–Paradigm Shift or Passing Fad?
UVM Names Robert Larner, MD, College of Medicine
Nov 27, 2017 | Laboratory Management and Operations, Laboratory News, Laboratory Operations, Laboratory Pathology, Laboratory Testing, Management & Operations, News From Dark Daily
November workshop to teach Clinical Lab 2.0 to forward-thinkers among clinical laboratories, IVD manufacturers, and lab IT vendors offered many examples where clinical laboratory diagnostics can add value and improve patient outcomes
DATELINE: ALBUQUERQUE, New Mexico—Here in this mile-high city, a special Project Santa Fe Workshop devoted to teaching the principles of Clinical Lab 2.0 attracted an impressive roster of innovators and forward-thinkers in clinical laboratory medicine. In attendance were leaders from a select number of the nation’s first-rank health systems and hospitals, along with executives from In Vitro diagnostics (IVD) manufacturers, lab IT companies, other lab service companies, attendees from the Centers for Disease Control and Prevention, and from institutions in Canada, Germany, Israel, India, and the UK.
Their common goal was to learn more about the emerging clinical and business model for medical laboratories known as “Clinical Lab 2.0.” A key objective of the workshop was to help those lab leaders in attendance develop strategic action plans for their own lab organizations, so as to take advantage of the insights coming from the vast information streams generated by their clinical laboratories. These services would be in support the evolving needs of health systems, hospitals physicians, and health insurers to more effectively provide integrated patient-centered clinical care.
Medical Laboratories Can Use Clinical Lab 2.0 as a Path to Adding Value
Clinical Lab 2.0 is the clinical and business model of the future for medical laboratories, assert the developers of this concept. “Clinical Lab 2.0 describes the attributes needed by all medical laboratories that want to succeed in a healthcare system organized to provide precision medicine, keep people out of hospitals, and where providers—including labs—are reimbursed based on the value they provide,” stated Khosrow Shotorbani, CEO of TriCore Reference Laboratories, one of the organizers of the Project Santa Fe Clinical Lab 2.0 Workshop.
“Clinical Lab 2.0 is the path medical labs will need to follow if they are to continue providing relevant lab testing services and generate the reimbursement necessary for them to maintain a high level of clinical excellence and financial stability going forward,” he added. “This is the next generation of medical laboratory organization and operation.”
Lab 1.0 Was Lab Clinical/Business Model for 50 Years
For more than 50 years, Clinical Lab 1.0 was the model for labs,” noted James Crawford, MD, PhD, Executive Director and Senior Vice President of Laboratory Services at Northwell Health Laboratories and an organizer of the Project Santa Fe Clinical Lab 2.0 Workshop. “Lab 1.0 is transactional, focusing on generating high quality analytical data on specimens received, but without assembling these data into integrative clinical care programs. In the simplest sense, Clinical Lab 1.0 focused on generating ever-greater numbers of specimens to drive down average cost-per-test, while maximizing revenue in a fee-for-service system.

This chart shows the attributes of Clinical Lab 1.0 and compares those to the attributes of Clinical Lab 2.0. Lab 1.0 is transactional and based on increasing test volume to lower costs and maximize fee-for-service revenue. Clinical Lab 2.0 is integrative in ways that add value to lab testing services. (Graphic copyright Project Santa Fe.)
“But fee-for-service payment is going away,” he said. “Increasingly, clinical laboratories will be paid based on the value they provide. This payment can be in the form of bundled reimbursement, as a per-member-per-month payment, or as a share of the budgeted payment made to a health system, an accountable care organization (ACO), or a multispecialty provider network. As these alternative forms of provider payment become dominant, to earn a fair share of reimbursement, all medical laboratories will need a clinical strategy to deliver lab testing services that measurably contribute to improved patient outcomes while reducing the overall cost of care. This requires looking at medical laboratories’ contribution to effective delivery of the full dollar of the healthcare spend, not just the three-cents-on-the-dollar representing laboratory testing.”
Innovators in Clinical Laboratory Industry Identify New Ways to Add Value
There are already a handful of innovative clinical laboratory organizations that have clinical experience in moving past the Lab 1.0 paradigm of reporting an accurate test result within the accepted turnaround time. Leaders within these labs are collaborating with physicians and frontline care givers specifically to help them better utilize lab tests in ways that directly improve the speed and accuracy of the overall diagnostic sequence, as well as achieving therapeutic optimization as rapidly as possible. These collaborations are tracking the improvement in patient outcomes while demonstrating how better use of lab tests can lower the total cost per episode of care.
During the Clinical Lab 2.0 workshop, case studies were presented demonstrating how clinical laboratory leaders are taking the first steps to practice Clinical Lab 2.0 so as to achieve added value with medical laboratory tests. The case studies included:
· A project to improve diagnosis and treatment of sepsis at Geisinger Health System.
· A project at Henry Ford Health to collaborate with physicians to more appropriately utilize lab tests and build consensus in support of a new lab test formulary.
· A multi-hospital initiative at Northwell Health to collaborate with physicians and nurses in the use of creating testing to make earlier, more accurate diagnoses of acute kidney injury during inpatient admissions, and better guide decisions to treat.
· A partnership involving TriCore Reference Laboratory and certain health insurers in New Mexico where the laboratory—using lab test data (some generated by emergency room testing) and other clinical data—alerts the insurers to women who are pregnant, thus allowing the insurers to provide timely guidance to the women’s care teams with the goal of improving prenatal care.

The Project Santa Fe Clinical Lab 2.0 Workshop convened on November 13-14 in Albuquerque, N.M. A broad spectrum of innovative professionals from the five Project Santa Fe member laboratories (above) were there to teach the lessons learned from their first successful efforts to collaborate with physicians and create added value from medical laboratory diagnostics. Other attendees included progressive lab leaders from several of the nation’s most prominent health systems, along with thought leaders from the IVD, lab software, and lab association sectors. (Photo copyright Project Santa Fe.)
Project Santa Fe Workshop: A Well-Attended Lab ‘Think Tank’
Participants attending the Clinical Lab 2.0 workshop included hospital lab administrators, pathologists, and clinical laboratory industry executives. The importance of this workshop is reflected in the educational grants and financial support provided by leading in vitro diagnostics manufacturers, lab IT companies, and other lab industry vendors. The lab industry vendors included:
· Abbott Laboratories
· ARUP Laboratories
· Beckman Coulter
· DiaSorin
· MedSpeed
· Roche Diagnostics
· Siemens Healthineers
· Sysmex
Also providing educational grants and similar support were:
· American Clinical Laboratory Association
· CAP Today
· Centers for Disease Control and Prevention
· Mayo Medical Laboratories
· The Dark Report
Project Santa Fe was launched in 2016 by clinical lab leaders from five of the nation’s most respected integrated health systems:
· TriCore Reference Laboratories;
· Henry Ford Health;
· Geisinger Health;
· Kaiser Permanente Northern California; and,
· Northwell Health.
Described as a think-tank venture, the organizers are committed to implementing projects that demonstrate how lab tests can be used in ways that add value, and then publish the resulting projects, along with data about improved patient outcomes and reductions in healthcare costs, in peer-reviewed journals. Multi-institutional studies will be required to validate the findings and outcomes from the added-value clinical collaborations initiated at the different medical laboratory organizations participating in Project Santa Fe.
Another primary goal is to share the lessons learned from these innovative projects with other like-minded pathologists, lab administrators, and lab managers. In May, Project Santa Fe organizers led a one-day workshop to teach Clinical Lab 2.0 at the Executive War College on Laboratory and Pathology Management. The workshop in Albuquerque on November 13-14 was the second learning opportunity available to medical laboratory professionals. A November 2018 workshop is planned.
—Robert L. Michel
Related Information:
Project Santa Fe Workshop
Improving American Healthcare through “Clinical Lab 2.0”: A Project Santa Fe Report
Laboratory 2.0: Changing the Conversation
CEO Describes Characteristics of the Clinical Lab 2.0 Model: Five Health System Labs Using Project Santa Fe To Demonstrate Value
Moving to Clinical Lab 2.0: Deliver More Value! Get Paid More Dollars!
Lab Innovators Advocate Need for Clinical Lab 2.0: Lab 1.0 Is the Low-Paid Commodity Lab, While Lab 2.0 Gets Paid More for the Value It Contributes
Using the Laboratory Value Pyramid and Clinical Lab 2.0 to Position Your Lab to Add Value in the Era of Population Health, Precision Medicine, and Value-Based Payment











