From some adverse events, clinical laboratory tests may be integrally tied to delays in treatment and faulty care management events that cause patient harm and deaths
Adverse patient safety events are increasing according to The Joint Commission (TJC), a nonprofit US-based organization that accredits tens of thousands of US healthcare organizations and programs. Its 2023 annual report on “sentinel events”—patient safety breaches that can inflict serious harm or death—found that these events jumped by 78.1% from 2020 to 2021 and by 19.3% from 2021 to 2022. These are significant increases. What effect do they have on healthcare providers?
Of particular interest for clinical laboratory managers and pathologists is one finding by TJC involving “delay in treatment” as an adverse event. TJC determined that the cause of 38% of these episodes was due to “missed diagnoses.” It is likely that clinical laboratory test results have some role in this type of adverse event. Thus, this is a potential opportunity for medical lab leaders to add value by helping hospital administrators and physicians reduce the number adverse events tied to missed diagnoses.
Another consequence for hospitals and providers is lost revenue. Medicare will not reimburse care to providers when a medical error, fall, surgery on wrong site, or other “never event” happens, including misdiagnoses due to inaccurate clinical laboratory testing.
Thus, since patient safety is a priority and providers are tasked with reducing medical errors, The Joint Commission’s Office of Quality and Patient Safety has developed the Sentinel Event Database for tracking adverse patient safety events.
The de-identified and aggregate patient data includes “causes and outcomes of sentinel events, is analyzed yearly to help the nation in general—and accredited organizations in specific—gain insight into causes of sentinel events and develop mitigating strategies to prevent harm to individuals under their care,” according to The Joint Commission’s report, “Sentinel Event Data 2022 Annual Review.”
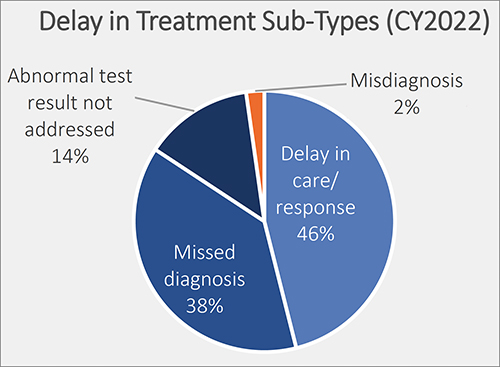
This graphic taken from The Joint Commission’s annual 2023 report on sentinel events shows the percentage of delays in treatment broken out by type. Delays in treatment is the second most common sentinel event, exceeding wrong surgery and medication management, according to The Joint Commission’s report. Only falls exceed delays in treatment, and since 80% of a patient’s health records are clinical laboratory test results, they could play a role in each of these adverse patient safety events. (Graphic copyright: The Joint Commission.)
Most Common Sentinel Events
According to The Joint Commission’s report, “Of reviewed sentinel events in 2022:
- “20% resulted in patient death,
- “6% in permanent harm or loss of function,
- “44% in severe temporary harm, and
- “13% in unexpected additional care/extended stay.
“Sentinel events resulting in death were most commonly associated with:
- “patient suicide (24%),
- “delays in treatment (21%), and
- “patient falls (11%).
“Events resulting in severe temporary harm were most commonly associated with patient falls (62%).”

The Joint Commission’s annual 2023 report on sentinel events shows the top 10 sentinel event types in 2022. According to the report, delay in treatment, which could be caused by inaccurate clinical laboratory tests, moved from fourth place in 2018, to third place in 2021, and is now in second place. (Graphic copyright: The Joint Commission.)
National Quality Forum ‘Never Events’
In 2001, Ken Kizer, MD, founding President and CEO of the National Quality Forum (NQF), coined the phrase “never events” to describe often fatal and usually preventable medical errors. Since then, the list of never events, according to PSNet, a national web-based resource of the Agency for Healthcare Research and Quality (AHRQ), has grown to include 29 “serious reportable events” in seven categories:
- Surgical or procedural events
- Product or device events
- Patient protection events
- Care management events
- Environmental events
- Radiologic events
- Criminal events
Listed under “Care management events” is “Patient death or serious injury resulting from failure to follow up or communicate laboratory, pathology, or radiology test results.”
In “Hospitals Take Steps to Drive Down Medical Errors in Their Emergency Departments,” Dark Daily wrote how one common source of errors in emergency rooms is lack of information that includes timely access to radiology or clinical laboratory test reports. Moreover, formal process improvement projects to identify and eliminate the sources of errors in emergency rooms can involve the hospital laboratory.
As medical laboratory managers know all too well, the fast-paced environment of a busy emergency room often contributes to errors such as hemolyzed specimens, a delay in transporting the specimen from the ER to the lab, or incomplete patient information on the laboratory test request form as it is received in the hospital laboratory.
‘Stuff of Nightmares‘
In an article he penned for Chicago-based thought leadership and advisory company 4Sight Health, longtime healthcare writer David Burda commented on The Joint Commission’s sentinel events report. He wrote: “Two things scared me from this eight-page report. First, The Joint Commission is publishing statistics and trends in reported sentinel events. How many go unreported? Second, surgeons still are performing the right procedure on the wrong patient or the wrong procedure on the right patient? Isn’t there a checklist to prevent this? The stuff of nightmares.”
Burda went on to note that “88% of the sentinel events in 2022 happened at accredited hospitals, and 90% were self-reported by accredited organizations. Patients, patients’ families, or current/former employees reported 10% of the sentinel events to The Joint Commission.”
The rapid rise of patient adverse safety events should be a concern for all sectors of the healthcare industry. Clinical laboratory managers should be especially concerned since nearly every medical decision precedes or follows a medical laboratory test.
—Ashley Croce
Related Information:
Sentinel Event Data 2022 Annual Review
CMS Improves Patient Safety for Medicare and Medicaid By Addressing Never Events
Quick Safety 52: Advancing Safety with Closed-Loop Communication of Test Results
New Sentinel Event Data Available for 2022
10 Most Common Sentinel Events of 2022: Joint Commission
Burda on Healthcare: Scared Healthy
Hospitals Take Steps to Drive Down Medical Errors in Their Emergency Departments



