Demand for a rapid, accurate diagnostic solution to combat Ebola is motivating research teams in many countries to develop solutions that can be put to immediate use
In West Africa, the outbreak of Ebola in several countries motivated researchers in Germany to develop a fast, accurate, and inexpensive test that could be performed in patient care settings without the need for a centralized medical laboratory.
In these West African countries, lack of electricity and reliable cold storage or diagnostic equipment handicaps clinical laboratory technicians who are testing patients for the Ebola virus. A new test developed by researchers at the German Primate Center (DPZ) in Göttingen, Germany, cuts the time to answer an Ebola diagnosis to just 15 minutes. It requires no electricity and is portable. Previously, the fastest Ebola diagnostics test took three hours to get results and required transporting samples to often-distant medical laboratories.
Suitcase Lab is a Self-Contained Mobile Medical Laboratory
The DPZ Diagnostics-in-a-Suitcase kit is a complete field diagnostics laboratory. It was developed by infectious disease researcher Ahmed Abd El Wahed, D.V.M., Ph.D., with assistance by Christiane Stahl-Hennig, D.V.M., Ph.D., head of the DPZ Unit of Infection Models.
“The early detection of Ebola infected patients will lead to a more effective virus control, since medical staff can identify and isolate confirmed Ebola cases more rapidly,” stated Stahl-Hennig in a DPZ press release. “In remote field hospitals, resources such as electricity and cold storage are often in short supply,” added Ahmed Abd El Wahed, “The Diagnostics-in-a-Suitcase will, therefore, contribute to a better management during the Ebola-outbreak.”

Pictured above is infectious disease researcher Ahmed Abd El Wahed, D.V.M., Ph.D. and Christiane Stahl-Hennig, D.V.M., Ph.D., head of the DPZ Unit of Infection Models, with their Diagnostics-in-a-Suitcase mobile lab. (Photo copyright German Primate Center. Credit: Karin Tilch, DPZ.)
Suitcase Lab Provides Speedy Diagnosis Using RPA Technology
The DPZ kit uses TwixtDx’s Recombinase Polymerase Amplification (RPA) technology to detect Ebola. RPA was adapted for use in diagnosing Ebola by Manfred Weidmann, M.D, Senior Lecturer at the University of Stirling, and Frank Hufert, M.D., Professor of Virology at the University Medical Center Göttingen.
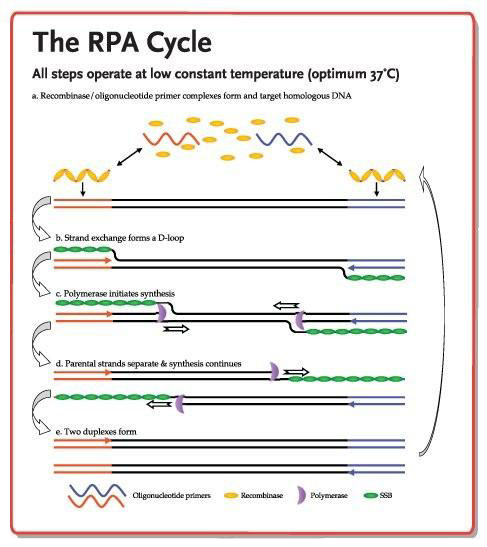
This diagram of the RPA cycle is licensed under a Creative Commons Attribution 3.0 United States License to TwistDx Ltd.
RPA test kits surpass the Ebola detection capabilities of both Roche’s LightMix® Ebola Zaire rRT-PCR Test and the U.S. Navy’s test technology developed for use at a new high-tech lab in Monrovia, Liberia.
The Roche test gets results within three hours, according to a report published in the International Business Times, while the U.S. Navy’s technology takes three to five hours for time to answer, according to a WHO report.
These two tests use a real-time polymerase chain reaction (PCR) technique that detects and amplifies Ebola’s unique RNA. This diagnostic test requires blood samples to be collected in the field and transported, often long distances, to a clinical laboratory, which presents safety issues, noted the DPZ press release.
The RPA-based Diagnostic-in-a-Suitcase test contains all reagents and equipment needed to detect the Ebola virus at point-of-need. It operates on an integrated solar panel and power pack. It can be utilized in the field, eliminating the risk of long-distance transporting of Ebola specimens.
RPA tests are as accurate as PCR-based tests. They work at a constant temperature, so no rapid heat-cycling equipment is required. And, reagents used in RPA tests are cold chain independent, which allows them to be used and transported at ambient temperatures.
DPZ’s innovative test kit is in field trials in Guinea, in collaboration with the Institut Pasteur de Dakar, Senegal, the Public Health Institute of Guinea, the United Kingdom’s University of Stirling, Robert Koch Institut, and TwistDx Ltd.
Rapid Ebola Technologies May be Adapted to Other Diagnostic Uses
This example from Germany shows how one team could miniaturize existing diagnostic technologies to fit in a suitcase and function in developing countries where the environment is tough on clinical laboratory testing solutions. As such systems prove themselves, it becomes easier for companies to then adapt these systems to meet the regulatory requirements for clinical use in developed nations. From that perspective, this technology development/demonstration pathway shows one more channel where diagnostic technologies can prove themselves, and then move “up market” for true global distribution and clinical use.
—Patricia Kirk
Related Information:
Suitcase Laboratory for Rapid Detection of Ebola
Fast, Simple Diagnostic Test Specific to 2014 Ebola Outbreak
Bandits in Guinea Steal Blood Samples Believed to Be Infected with Ebola



