Analysis of almost 3 million newborn blood samples found that tens of thousands of specimens were not screened promptly for rare but deadly disorders, leading to patient harm in some cases
State-mandated newborn testing has come under increased media scrutiny following the discovery that delays in reporting the clinical laboratory test results had resulted in harm to some children with genetic diseases. One source of problems is some hospitals fail to promptly submit specimens from babies to their state’s newborn testing laboratory.
In Wisconsin, pathologists and medical laboratory and laboratory managers probably know the story of Colton Hidde because of news stories about his case. When Karen and Mike Hidde brought their newborn baby Colton home from the hospital after his birth in October 2012, they had no idea that he would soon be close to death. He appeared to be a normal newborn. But he was not, and the Hiddes didn’t find out that he had a rare and life-threatening genetic defect until they rushed him back to the hospital less than 24 hours after bringing him home.
Newborn Test Results Not Reported on Time
The story of the Hidde family is one of several in a remarkable series of articles on newborn screening (NBS) published in the Milwaukee Journal Sentinel beginning November 16 and continuing even now. For the series, reporters analyzed almost 3 million NBS samples and found that tens of thousands of blood samples from babies across the country were not screened promptly for rare, but deadly disorders.
In the series titled Deadly Delays, the newspaper reported that many hospitals ignore regulations that require them to send newborns’ blood samples to labs promptly, but these hospitals suffer no consequences when the samples are late. The newspaper also reported that medical laboratory administrators and public health officials in many states fought to hide the performance of some hospitals.
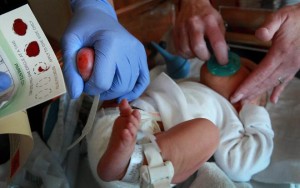
Pictured above is the collection of a specimen as part of state-mandated newborn screening (NBS) programs. News coverage by the Milwaukee Journal Sentinel revealed that delays and other problems with the NBS program have harmed some newborn babies. Clinical laboratories at hospitals across the nation should be taking steps to ensure that NBS samples are sent promptly to their state’s NBS lab facility. (Photo by Kristyna Wentz-Graff and copyright Milwaukee Journal Sentinel.)
For nearly 15 years, federal regulators and public health officials have discussed the need to standardize newborn screening (NBS) systems throughout the country. However, little action has been taken beyond increasing the number of conditions tested, the Journal Sentinel reported.
Senate Passes Bill to Address Problems in Newborn Screening
As result of this media coverage, the U.S. Senate passed a bill, S. 1417, the Newborn Screening Saves Lives Reauthorization Act, on January 29 to revise NBS regulations. A companion bill may be considered in the house this session, said Cynthia Pellegrini, Senior Vice President for Public Policy and Government Affairs for the March of Dimes.
“No baby should die or suffer the devastating health consequences of a condition that could have been treated or prevented if identified through newborn screening,” stated March of Dimes President Jennifer L. Howse, Ph.D.
Another result of the news stories was that the March of Dimes, the Association of Public Health Laboratories (APHL) , and the American Hospital Association (AHA) called on medical laboratories in hospitals to make improvements in NBS procedures. The Milwaukee Journal Sentinel published a story on these developments.
American Hospital Association Issued a Quality Advisory
The AHA issued a quality advisory, asking its member hospitals to review their NBS performance. The APHL asked lab directors in all 50 states to read the series of news stories, saying it “exposes gaps in the system that could be addressed if handled carefully.” Newspapers in many of the poor-performing states reported on delays in their states too.
The Milwaukee Journal Sentinel identified the fact that many hospital labs are closed on weekends and holidays, potentially causing delays in testing of two or three days for some babies. Also, hospitals should send samples via overnight delivery service, but it still takes days for hundreds of thousands of samples to arrive at labs for testing, the newspaper reported. “In some cases, hospitals are sending samples through the U.S. mail even when a courier service is arranged and paid for by the state,” the newspaper reported. The medical laboratories doing this state-mandated testing also often run NBS samples in batches rather than upon arrival, causing further delays.
One in Every 800 Babies Is Born with a Potentially Severe Condition
About one in every 800 babies is born with a potentially severe or deadly condition that could be treated and managed if the child were tested properly, the Journal Sentinel said, adding, “These babies often appear healthy at birth but can become extremely sick within days.”
In a blog about news stories on this topic, which was published on the Massachusetts Institute of Technology’s Knight Science Journalism program Website, Blogger Paul Raeburn wrote:
- At one hospital in Phoenix, “70% of samples took five or more days to get to the state lab just seven miles away.”
- Last year in New York, only 60% of samples arrived at the state lab within 48 hours, as required by law.
- Most state-run programs do not follow guidelines issued in 2005.
South Carolina Ranks Near Bottom for Timely Delivery of NBS Samples
In a recent follow-up article, the newspaper reported that South Carolina became the 30th state to release information about how specific hospitals perform NBS. “The state ranks among the worst in the nation as to how quickly hospitals send babies’ blood samples to state labs for testing.. Last year, 34% of newborn screening samples took five or more days to get to the state lab for testing—a percentage that’s more than double that of other poor-performing states, including Arizona and Texas. More than a dozen of the state’s hospitals had at least half of babies’ blood samples delayed.”
This issue is of critical importance to hospital clinical laboratories and health systems because the media coverage of this problem shows a disregard for important patient-care and liability issues involved. In addition, patients, health plans, employers, and government payers all have higher expectations about what constitutes quality patient care than they had in the past, and hospitals are being ranked on their ability to meet and exceed demands for increased patient satisfaction.
Media Coverage Highlighted a Problem that Endangered Newborns
Few hospitals have paid much attention to the turnaround time for NBS, but that may now change thanks to the Milwaukee Journal Sentinel’s news coverage, which has raised public awareness of healthcare practices that endanger newborns. Its reporting also showed that hospitals in some states, including Wisconsin, were not monitoring the delays in specimen submissions and that state officials were not notifying hospitals about excessive delays.
At a more strategic level, pathologists and medical laboratory managers should understand at least two implications of this situation. First, when newborn babies fail to get timely treatment for genetic diseases because their NBS testing results were not provided to the care team on a timely basis, it can trigger unfavorable news coverage for the hospital where the child was born. Second, advances in molecular diagnostics and genetic testing make it likely that newborn screening programs will be expanded to include testing for additional types of diseases and genetic conditions. That will also raise the risk factor for any hospital that fails to submit specimens on a timely basis to the lab doing the newborn screening.
—Joseph Burns
Related Information:
A Journal Sentinel Watchdog Report: Deadly Delays
Testing delay puts newborn’s life at risk
Wisconsin hospitals to get regular reports on newborn screening performance
Journal Sentinel’s Deadly Delays: Another striking failure of U.S. healthcare
SC hospitals slow in getting newborn tests to lab
Newborn Screening Saves Lives Reauthorization Act Passed by Senate



