Pathologists and clinical laboratory managers can expect to see an expansion of genetic testing in the wake of the Supreme Court’s decision in the Myriad case
Pathologists and clinical laboratory professionals got a major victory on June 13. That’s when the United States Supreme Court (SCOTUS) ruled 9-0 to end the 30-year-old practice of awarding patents on human genes. The unanimous decision invalidates certain hotly contested patents held by Myriad Genetics, Inc., (NASDAQ: MYGN) on the BRCA1 and BRCA2 genes.
Moreover, this Supreme Court decision also opens the doors to other medical laboratories to develop their own diagnostics around the BRCA genes and compete for breast-cancer testing market share.
It took only a few hours after the ruling was made public for several clinical lab companies to announce their BRCA gene tests for breast cancer. These labs included Bio-Reference Laboratories, Inc. (NASDAQ: BRLI), Ambry Genetics, Quest Diagnostics Incorporated (NYSE: DGX), and University of Washington for starters.
Natural Genes Are No Longer Patentable
In short, the result of the unanimous decision is that:
1. Isolated genomic DNA (gDNA) is not patent-eligible under section 101 of the Patent Act; and
2. complementary DNA (cDNA) is patent-eligible under section 101 of the Patent Act.
“It’s such a win for patients,” stated Elizabeth Chao, M.D., geneticist and Chief Medical Officer at Ambry Genetics, in a story published at Nature.com. Ambry is a medical diagnostics company in Aliso Viejo, California.
“Today, the court struck down a major barrier to patient care and medical innovation,” observed Sandra Park, senior staff attorney with the Women’s Rights Project of the American Civil Liberties Union (ACLU), in a report published at abcnews.go.com. The ACLU represented the plaintiffs in the lawsuit.
An Epic Journey through the Courts
The suit, Association for Molecular Pathology v. Myriad Genetics, Inc. (2013), challenged the validity of gene patents in the United States. Specifically, it challenged certain patents owned or controlled by Myriad, including patents on the BRCA1 and BRCA2 genes.

In the case of the Association for Molecular Pathology v. Myriad Genetics, Inc., the Supreme Court justices voted 9-0 to strike down Myriad’s BRCA1 and BRCA2 patents that covered natural isolated DNA. At the same time, the court held that complementary DNA is patent-eligible. Meanwhile, at the U.S. Patent and Trademark Office, the Deputy Commissioner for Patent Examination Policy, Andrew Hirshfeld, issued a memo to the patent examining corps instructing examiners to “reject product claims drawn solely to naturally occurring nucleic acids or fragments thereof, whether isolated or not, as being ineligible subject matter under 35 U.S.C. Section 101.” (photo from Wikimedia.org)
Myriad’s exclusivity business model allowed it to attract investors. It also meant that, without competition, the company could: 1) charge a premium price—US$4,000—for its BRCA gene test; and, 2) patients had no recourse for a second opinion that would be based on another laboratory’s version of the BRCA gene test.
During the hearing at the Supreme Court, the parties offered these basic arguments:
• Proponents: gene patents encourage investment in biotechnology and promote innovation in genetic research.
• Opponents: gene patents stifle innovation by preventing others from conducting cancer research; gene patents limit options for cancer patients, and gene patents are invalid because isolated genes are a product of nature and not inventive.
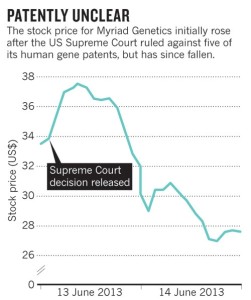
Dip in Myriad Stock Price Reflects Investor Uncertainty following Ruling
Despite the high court’s ruling, Myriad still has more than 500 claims covering its tests for breast-cancer genes, a story published in Genomics Law Report pointed out. This may have sustained investor confidence following the ruling—at least initially, according to the Nature.com story.
During the breaking news cycle, Myriad stock climbed about $5, peaking at almost $38 by the end of the day. That’s according to Forbes.com contributor, Dan Munro. However, once other medical laboratory firms announced plans to launch their own tests,. Myriad’s shares were down by almost 20%.
Revenue from Myriad’s BRCA test in the third quarter of FY2013 reached $115.4 million, according to a company press release. This represented a 9% increase over the same period of the prior year and 74% of Myriad’s total revenue for the quarter.
Now faced with competition, Myriad will be fighting to maintain market share. Competitors may offer the BRCA test for as much as half the price of the Myriad test, according to the Nature story.
Pathologists and clinical laboratory managers should expect an expansion of genetic diagnostic testing following the Supreme Court’s long-awaited decision in Association for Molecular Pathology v. Myriad Genetics, Inc. The justices voted 9-0 to strike down Myriad’s BRCA1 and BRCA2 patents that covered natural isolated DNA. At the same time, the court held that complementary DNA is patent-eligible. Would-be competitors, as well as patients and their advocates, greeted the decision enthusiastically. Biotechnology investors, on the other hand, were left uncertain, as Myriad’s stock began a decline. Meanwhile, at the US Patent and Trademark Office, Deputy Commissioner for Patent Examination Policy, Andrew Hirshfeld, issued a memo to its examining corps instructing examiners to “reject product claims drawn solely to naturally occurring nucleic acids or fragments thereof, whether isolated or not, as being ineligible subject matter under 35 U.S.C. Section 101.”
Myriad Retains Significant Competitive Advantage in BRCA Testing
Myriad’s extensive proprietary database of 16,000 mutations gives it a significant competitive advantage, according to Amanda Murphy, CFA, healthcare analyst at William Blair & Company. This allows Myriad to identify the mutation and the associated breast cancer risk 98% of the time, wrote Murphy in an equity research briefing. By contrast, the public database currently has only 1,000 mutations.
Despite some debate over the issue, Murphy wrote that competing labs, because of their reliance on the more limited public database, could miss mutations about 20% to 40% of the time. Therefore, its unique, proprietary database of associations between mutations and clinical outcomes appears to put Myriad ahead of competitors in its ability to interpret breast cancer test data.
The key for pathologists and clinical laboratory managers is that the SCOTUS decision is poised to broaden the genetic-testing market. This will be particularly true for the development of multi-gene assays as part of a single diagnostic test. At a minimum, it will open the doors to allow other clinical laboratories to work within the natural BRCA1 and BRACA2 genes to make useful diagnostic information available to patients.
—Pamela Scherer McLeod
Related Information:
Myriad BRCA Patents Nixed: What Happens Next?
Angelina Jolie, breast cancer testing, Myriad Genetics and the Supreme Court
Myriad, Finally: Supreme Court Surprises by not Surprising
Myriad Genetics shares rise to three-year highs
Angelina Jolie star power extends to stock
Change to gene patents leaves US biotech in a lather.



