By placing this low-cost, disposable device developed at the University of Wisconsin-Madison on their arms or abdomens, patients can collect their own blood at home in minutes
For more than two years, the nation’s media have been captivated by Theranos CEO Elizabeth Holmes’ vision of offering patients who need blood tests a finger stick collection instead of a venipuncture. Meanwhile, in research labs across the nation, there are credible efforts to develop ways to collect medical laboratory test specimens that require no needles at all.
On such effort may soon enter the market. It is an innovative, needleless blood-collection device called HemoLink developed by a research team at the University of Wisconsin-Madison. Users simply place a device with the diameter of a golf ball against their arms or abdomens for two minutes. During that time, the device draws blood from capillaries into a small container. Patients would then mail the tube of collected blood to a medical laboratory for analysis.
This non-threatening device is ideal for children. However, patients who require recurrent blood tests to monitor health conditions would also benefit, as it would save them frequent trips to clinical laboratories for blood draws using traditional needle-stick methods.
How the HemoLink Device Works
In a process known as “capillary action,” HemoLink leverages microfluidics to create a slight vacuum that pulls blood from capillaries though tiny channels in the skin into a small tube, noted a Gizmag report. The device collects 0.15 cubic centimeters of blood, which is enough to test for cholesterol, infections, cancer cells, blood sugar and other conditions.
Pathologists and clinical laboratory professionals will be watching the eventual launch of HemoLink to learn how its developers have overcome the problems affecting lab test accuracy that can be caused by the interstitial fluid that often accompanies capillary blood when such specimens are collected. How the lab test technology used by Theranos addresses this same problem has been an ongoing point of interest among medical laboratory professionals.
Capillary Blood Collection Device Developed at University of Wisconsin-Madison
Tasso Inc., the medical technology startup that developed HemoLink, was co-founded by three former University of Wisconsin-Madison microfluidics researchers:
• Ben Casavant, Ph.D., Vice President of Operations and Engineering at Tasso,
• Erwin Berthier, Ph.D., Vice President of Research & Development and Technology, and,
• Ben Moga, M.S., President.
Casavant explained why microfluidic forces work: “At these scales, surface tension dominates over gravity, and that keeps the blood in the channel no matter how you hold the device,” he stated in the Gizmag report.
The project was funded with $3 million from the Defense Advanced Research Projects Agency (DARPA), the research arm of the U.S. Department of Defense (DOD).

Formerly microfluidics researchers at the University of Wisconsin-Madison, the three co-founders of Tasso, Inc., (left to right) Ben Casavant, Vice President of Operations and Engineering; Erwin Berthier, Vice President of R & D and Technology; and Ben Moga, President, conceived the concept of HemoLink in a coffee shop. (Photos copyright Tasso, Inc.)
Ensuring Stability of Blood Samples for Medical Laboratory Testing
The HemoLink device is inexpensive to manufacture and Tasso hopes to have the device available to consumers in 2016, reported Gizmag. That might, however, depend on whether the Tasso scientists can develop a method to ensure blood sample stability.
Currently, most blood samples for clinical laboratory testing require cold-chain shipping. According to the Gizmag report, Tasso scientists want to store blood samples at 140-degrees Fahrenheit for one week to ensure they will be fit for analysis when they arrive for processing by a clinical laboratory. Tasso plans to apply for U.S. Food and Drug Administration (FDA) clearance by the end of this year.
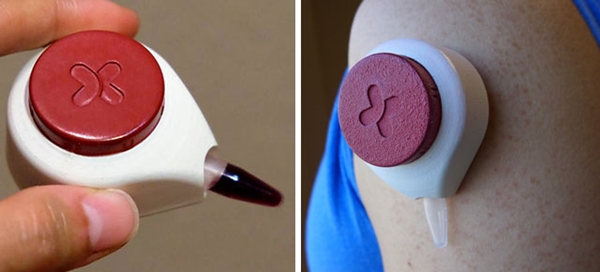
HemoLink, an inexpensive, disposable, needleless blood collection device, may be available to consumers by 2016. It uses a process called “capillary action” to draw blood into a collection tube. Users simply place it on their arm or stomach for two minutes, and then mail the tube to a medical laboratory for analysis. (Photos copyright Tasso, Inc.)
Highly Beneficial for Remote Blood Collection: Maybe Not So Good for Theranos?
HemoLink is good news for people who don’t like needle sticks as well as payers concerned with holding down healthcare costs. Further, if Tasso can succeed and win FDA clearance, it also could provide people everywhere in the world—even in remote locations—with a way to connect to central blood analysis laboratories and benefit from cutting-edge diagnostics.
“We have compelling data, a motivated management team, and an unmet clinical need in a growing market,” Moga declared in the Gizmag report. “Expanding home care by enabling safe and convenient blood draws for clinical diagnosis and monitoring is exactly the kind of innovation that improves outcomes without raising healthcare costs.”
But not all medical laboratory industry stakeholders would be happy with HemoLink’s introduction into the marketplace. This is potential disruptive technology for both clinical laboratories and the Silicon Valley bio-tech company Theranos, which has spent millions perfecting a method of running complex blood tests from a finger-stick blood sample, noted a USA Today report.
It would be ironic if the developers of HemoLink were able to iron out any kinks with their technology, obtain FDA clearance, and come to market during the next 24 months with a product that eliminated the need for both venipunctures and finger-stick sample collections for many types of medical laboratory tests. That would certainly steal some of the “disruptive thunder” from Theranos, which has spent the past two years promoting its vision of disruption to the clinical laboratory testing industry as it operates today.
—Patricia Kirk
Related Information:
New Sampling Device Promises to Make Blood Tests Needle-free
DARPA Takes a Stab at Needle-Free Blood Draws



Hi Mary,
I’m not sure how well it actually works – theranos have not released much information. They have also been given a cease trading notice in the last couple of days. My understanding of these devices is that they use a high density “cluster” of capillaries – which function as a needle. They probably would leave a slightly inflamed region – but I think the overall skin penetration is not as deep as needles e.g. accuchek.
Hope that Helps
I do not understand how this works. If it is pulling blood through the skin, would it not raise an area of blood also known as a hickey? Skin is avascular so how is it doing this? Can someone please explain some science behind this? I think this is a wonderful idea…but I would like to know more. Thanks